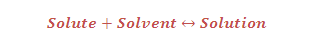Solubility:
Solubility of a substance is its maximum
amount that can be dissolved in a specified amount of solvent at a specific
temperature. It depends upon the nature of solute and solvent as well as
temperature and pressure.
Unit:
Unit of Solubility is gm/litre
or Mole/Litre
(A) Solubility of Solid in a Liquid:
Every solid does not dissolve in a given liquid. While sodium chloride and
sugar dissolve readily in water, naphthalene and anthracene do not. On the
other hand, naphthalene and anthracene dissolve readily in benzene but sodium
chloride and sugar do not.
(1)
It is clear observed that polar solutes dissolve in polar
solvents and non polar solutes in non-polar
solvents.
(2)
In general, a solute dissolves in a solvent if the intermolecular interactions
are similar in the two or we may say like dissolves like.
(3) Dissolution: When a solid solute is added to the solvent, some
solute dissolves and its concentration increases in solution. This process is
known as dissolution.
(4) Crystallisation: Some solute particles in solution collide with the
solid solute particles and get separated out of solution. This process is known
as crystallisation.
“A stage is
reached when the two processes (dissolution and crystallisation) occur at the same rate. Under such
conditions, number of solute particles going into solution will be equal to the
solute particles separating out and a state of dynamic equilibrium is reached.
At this stage the concentration of solute in solution will remain constant
under the given conditions, i.e., temperature and pressure. Similar process is
followed when gases are dissolved in liquid solvents.
(5) Saturated solution: Such a solution in which no more
solute can be dissolved at the same temperature and pressure is called a saturated solution.
(6) Unsaturated solution: The Solution in which more solute can be
dissolved at the same temperature.
(7) The solution which is in dynamic equilibrium with
undissolved solute is the saturated solution and contains the maximum amount of
solute dissolved in a given amount of solvent. Thus, the concentration of
solute in such a solution is its solubility.
Factors affecting
Solubility:
Earlier we have observed that solubility of one substance into another depends
on the nature of the substances. In addition to these variables, two other
parameters, i.e., temperature and pressure also control this phenomenon.
(1) Effect of temperature:
The solubility of a solid in a liquid is significantly affected by temperature changes.
Consider the equilibrium exist between dissolution and crystallisation. This, being dynamic equilibrium, must follow Le Chateliers Principle.
In general…….
(i) If in a nearly saturated solution,
the dissolution process is endothermic (Δsol H > 0), the solubility
should increase with rise in temperature and
(ii) If it is exothermic (Δsol H > 0) the
solubility should decrease. These trends are also observed experimentally.
(2) Effect of Pressure:
Pressure does not have any significant effect on solubility of solids in liquids.
It is so because solids and liquids are highly incompressible and practically remain
unaffected by changes in pressure.
(2) Solubility of gas in
Liquid:
Many gases dissolve in water. Oxygen dissolves only to a small extent in water.
It is this dissolved oxygen which sustains all aquatic life. On the other hand,
hydrogen chloride gas (HCl) is highly soluble in water. Solubility of gases in
liquids is greatly affected by pressure and
temperature.
Factors affecting
Solubility:
(1) Effect of Pressure:
The solubility of gases increase with
increase of pressure. For solution of gases in a solvent, consider a solution
is act as system and that system to be in a state of dynamic equilibrium,
i.e., under these conditions rate of gaseous particles entering and leaving the
solution phase is the same. Now increase the pressure over the solution phase
by compressing the gas to a smaller volume, this will increase the number of gaseous
particles per unit volume over the solution and also the rate at which the
gaseous particles are striking the surface of solution to enter it. The
solubility of the gas will increase until a new equilibrium is reached
resulting in an increase in the pressure of a gas above the solution and thus
its solubility increases.
Henry’s Law:
(1) The solubility of a gas in a liquid
is determined by several factors. In addition to the nature of the gas and the
liquid, solubility of the gas depends on the temperature and pressure of the
system.
(2) The
solubility of a gas in a liquid is governed by Henry’s law which states that the
solubility of a gas in a liquid is directly proportional to the pressure of the
gas.
(3) Dalton, a contemporary of Henry, also
concluded independently that the solubility of a gas in a liquid solution is a
function of the partial pressure of the gas. If we use the mole fraction of the
gas in the solution as a measure of its solubility, then: Mole fraction of the
gas in a solution is proportional to the partial pressure of the gas.
Or, partial pressure of the gas in solution = KH ´ mole fraction of the gas in solution
Here KH is Henry’s law constant
p = KH X
(Solute)
If we draw a graph between partial pressure of
the gas versus mole fraction of the gas in solution, then we should get a plot
of the straight line passing through origin.
Experimental result for the solubility of HCl gas in Cyclohexane
at 93 K the slope of line is the Henry’s law constant
Different gases have different KH
values at the same temperature. This suggests that KH is a function
of the nature of the gas. Table gives KH values of some common gases
at specified temperature
Values of Henry’s law constant (KH) for some selected
gases in water:
It is obvious from figure that the
higher the value of KH at a given pressure, the lower is the
solubility of the gas in the liquid. It can be seen from table that KH value for both N2 and O2 increases
with increase in temperature indicating that solubility of gases decreases with
increase of temperature. It is due to this reason that aquatic
species are more comfortable in cold waters rather than warm waters.
Application of Henry’s Law:
Henry’s law finds several applications in industry and explains some biological
phenomena Notable among these are:
(1) To increase the solubility of CO2 in
soft drinks and soda water, the bottle is sealed under high pressure.
(2) To minimize the painful effects accompanying
the decompression of deep sea divers, oxygen diluted with less soluble helium
gas is used as breathing gas.
(3) In lungs, where oxygen is present in air with
high partial pressure, haemoglobin combines with oxygen to form oxyhaemoglobin.
In tissues where partial pressure of oxygen is low, oxyhaemoglobin releases oxygen
for utilization in cellular activities.
(1) At High Pressure:
Scuba divers must cope with high concentrations
of dissolved gases while breathing air at high pressure underwater. Increased
pressure increases the solubility of atmospheric gases in blood. When the divers
come towards surface, the pressure gradually decreases. This releases the
dissolved gases and leads to the formation of bubbles of nitrogen in the blood.
This blocks capillaries and creates a medical condition
known as bends, which are painful and dangerous to life.
To avoid bends, as well as, the toxic effects
of high concentrations of nitrogen in the blood, the tanks used by scuba divers
are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(2) At Low Pressure: At high altitudes the partial pressure of
oxygen is less than that at the ground level. This leads to low concentrations
of oxygen in the blood and tissues of people living at high altitudes or
climbers. Low blood oxygen causes climbers to become weak and unable to think clearly,
symptoms of a condition known as anoxia.
(2) Effect of
temperature:
Solubility of gases in liquids decreases with rise in temperature. When dissolved,
the gas molecules are present in liquid phase and the process of dissolution can be
considered similar to condensation and heat is evolved in this process.
We have known that dissolution process involves dynamic
equilibrium and thus must follow Le Chatelier’s Principle. As dissolution is an
exothermic process, the solubility should decrease with increase of temperature.
ILLUSTRATIVE EXAMPLE (1): If N2
gas is bubbled through water at 293 K, how many millimoles of N2 gas
would dissolve in 1 litre of water. Assume that N2 exerts a partial
pressure of 0.987 bar. Given that Henry’s law constant for N2 at 293
K is 76.84 kbar.
SOLUTION: The
solubility of gas is related to its mole fraction in the aqueous solution. The
mole fraction of the gas in the solution is calculated by applying Henry’s law.
Thus,
As 1litre water contains 55.5 mol of it, therefore, if n represents number of moles of N2 in solution,