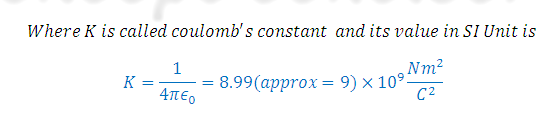Search This Blog
Showing posts with label STRUCTURE OF ATOM:. Show all posts
Showing posts with label STRUCTURE OF ATOM:. Show all posts
Thursday, June 18, 2020
What is the relation between Total energy (TE), Kinetic energy (KE) and Potential energy (PE) of Bohr's orbits?
Relation between Total energy (TE), Kinetic energy (KE) and Potential energy (PE):
Important conclusions:
(1) The minus sign for the energy of an electron in an orbit represents attraction between the +vely charged nucleus and negatively charged electron.
(2) Energy of an electron at infinite distance from the nucleus is zero.
(3) As an electron approaches the nucleus, the electrical attraction increases, energy of electron decreases and it becomes negative.
(4) Energy of an electron increases as the value of ‘n’increases i.e.
(5) Value of ‘n’ remaining unchanged, the amount of energy associated with an electron remains unaltered.
(6) Energy of electron in first, second, third and fourthorbit are –13.6, –3.4, –1.5, and –0.85 eV/atomrespectively.
(7) Although the energy of electron increases with increase in the value of ‘n’ (orbit), yet the difference of energy between successive orbits decreases. Thus E2 – E1 > E3 – E2 > E4 – E3 > E5 – E4 >, etc…
How to calculate energy of Bohr's orbits in term of Rydberg's constant
Which of the Hydrogen spectrum series found in visible range of spectrum?
Balmer series of Hydrogen spectrum found in visible range of spectrum while lymen series found in UV range of spectrum and remain series of spectrum found in Infra red of spectrum..
More over only first four line of Balmer series found in visible range and others line of Balmer series Lines also found infrared part of spectrum.
Friday, June 21, 2019
ENERGY IN BOHR'S ORBITS:
Total energy of (E) of an electron
revolving in nth orbit is equal to sum of kinetic
energy and Potential energy.
We know the
electron revolve around nucleus due balancing of two forces columbic and
centrifugal forces
This is the
famous Bohr’s equation applicable to Hydrogen like atoms or ions as He+1,
Li+2 , Be+3
etc.
The factor
(4 pi epsilon zero) is known as permittivity factor and its numerical value is
1.11268*10-10C2N-1M-2 ( In CGS Unit K= 1)
Pi= 22/7=
3.424, me=9.109 *10-31 kg, e = 1.602 *10-10 C and h=
6.626*10-34 j-s
Calculation of En
in SI Unit:
Bohr’s energy in
electron volt:
We know that,
1eV = 1.602 *10-19 J hence
Energy in term of Rydberg’s
Constant:
Relation between Total
energy (TE), Kinetic energy (KE) and Potential energy (PE):
Important conclusions:
(1) The minus sign for the energy of an electron
in an orbit represents attraction between the +vely charged nucleus and negatively charged electron.
(2) Energy of an electron at infinite distance
from the nucleus is zero.
(3) As an electron approaches the nucleus, the
electrical attraction increases, energy of electron decreases and it becomes
negative.
(4) Energy of an electron increases as the value
of ‘n’
increases i.e.
(5) Value
of ‘n’
remaining unchanged, the amount of energy associated with an electron remains
unaltered.
(6) Energy of electron in first, second, third and fourth orbit are –13.6, –3.4, –1.5, and
–0.85 eV/atom respectively.
(7) Although the energy of electron increases with
increase in the value of ‘n’ (orbit), yet the difference of energy between
successive orbits decreases. Thus E2 – E1 > E3 – E2
> E4 – E3 > E5 – E4 >,
etc….
Wednesday, February 20, 2019
HEISENBERG'S UNCERTAINTY PRINCIPLE:
"It is not possible to determine
simultaneously the exact position and exact moment of a particle as small as an
electron"
ILLUSTRATIVE EXAMPLE
(1): If error in position of an electron is 0.33 pm, what
will be the error in its velocity? (1 pm=10-12)
ILLUSTRATIVE EXAMPLE (2): If H+ (ion) is accelerated to a final velocity of
6.62×10+6 meter per second and error in velocity is 1% then
find uncertainty in position is?
ILLUSTRATIVE
EXAMPLE (3): Radius of nucleus
is the order of 10-13 cm (10-15 m) and thus on the basis
of Heisenberg's uncertainty principle .show that electron cannot exist within
the atomic nucleus?
SOLUTION:
ILLUSTRATIVE EXAMPLE (4): If uncertainty in position and momentum of electron are equal then prove that uncertainty in velocity is ...
ILLUSTRATIVE EXAMPLE (4): If uncertainty in position and momentum of electron are equal then prove that uncertainty in velocity is ...
SOLUTION:
ILLUSTRATIVE EXAMPLE (5):If uncertainty in momentum of an electron are three times of uncertainty in position then uncertainty in velocity of electron would be
ILLUSTRATIVE EXAMPLE (5):If uncertainty in momentum of an electron are three times of uncertainty in position then uncertainty in velocity of electron would be
SOLUTION:
ILLUSTRATIVE EXAMPLE (6): What is the uncertainty of Photon in position of wave length 500 A .If wave length is known to an accuracy of 1pm.
ILLUSTRATIVE EXAMPLE (6): What is the uncertainty of Photon in position of wave length 500 A .If wave length is known to an accuracy of 1pm.
SOLUTION:
ILLUSTRATIVE EXAMPLE (7): An electron is accelerated by (V) volt and following graph is obtained calculate the (V) voltage?
ILLUSTRATIVE EXAMPLE (7): An electron is accelerated by (V) volt and following graph is obtained calculate the (V) voltage?
SOLUTION:
ILLUSTRATIVE EXAMPLE (8): A electron having velocity 2×10+6 m/s has uncertainty in kinetic energy is 6.62/π×10-34 j, than calculate the uncertainty in position of electron in Anstrom .
ILLUSTRATIVE EXAMPLE (8): A electron having velocity 2×10+6 m/s has uncertainty in kinetic energy is 6.62/π×10-34 j, than calculate the uncertainty in position of electron in Anstrom .
SOLUTION:
ILLUSTRATIVE EXAMPLE (9): Two particles A and B are in motion .if the wave length associated with particle A is 5×10-8 m. Calculate the wave length associated with particle B if momentum is Half of A?
ILLUSTRATIVE EXAMPLE (9): Two particles A and B are in motion .if the wave length associated with particle A is 5×10-8 m. Calculate the wave length associated with particle B if momentum is Half of A?
SOLUTION:
ILLUSTRATIVE EXAMPLE (10): If uncertainty in position of an moving electron is equal to its de Broglie wave length, then its velocity will be completely uncertain. Explain?
ILLUSTRATIVE EXAMPLE (10): If uncertainty in position of an moving electron is equal to its de Broglie wave length, then its velocity will be completely uncertain. Explain?
SOLUTION:
ILLUSTRATIVE EXAMPLE (11): If the de Broglie wave length of a particle of mass (m) is 100 times of its Velocity. Then its value in term of its mass (m) and plank constant (h) is?
ILLUSTRATIVE EXAMPLE (11): If the de Broglie wave length of a particle of mass (m) is 100 times of its Velocity. Then its value in term of its mass (m) and plank constant (h) is?
Subscribe to:
Posts (Atom)