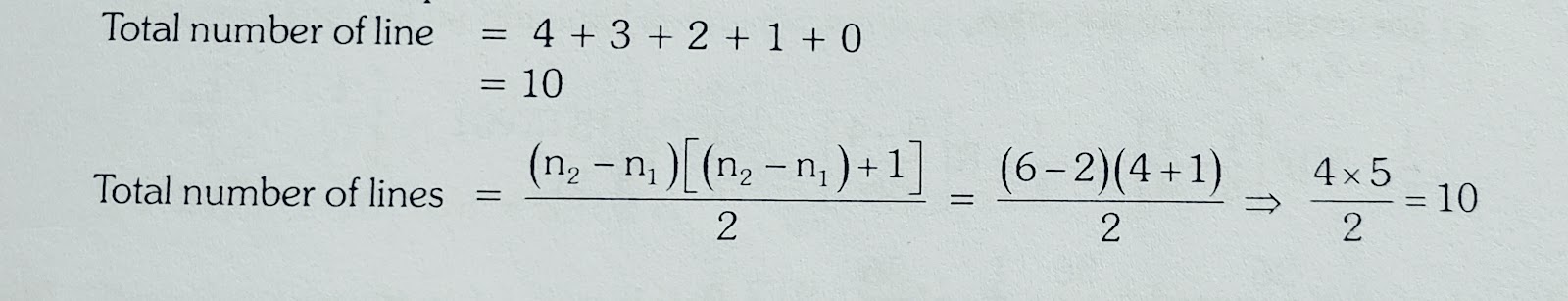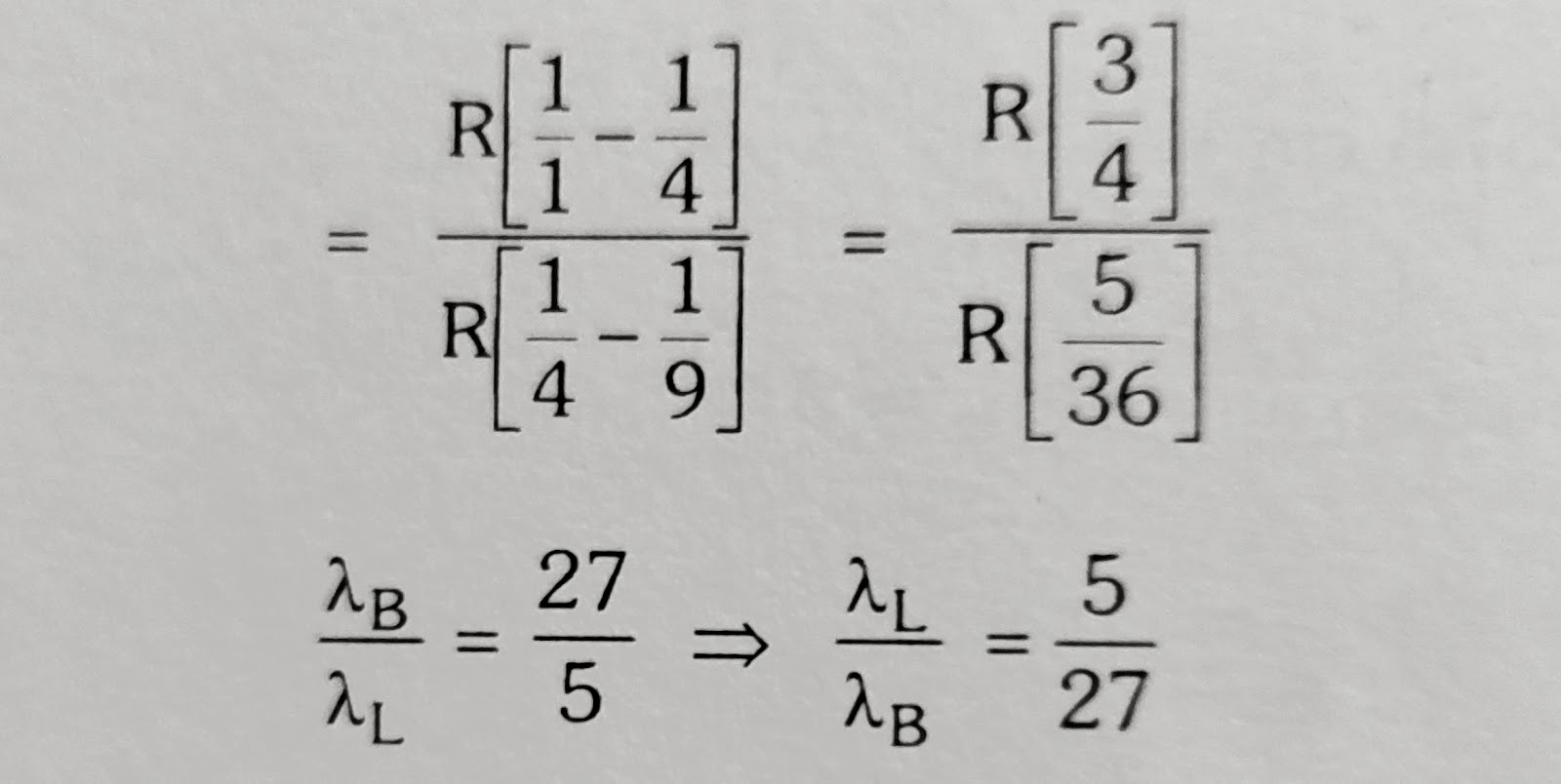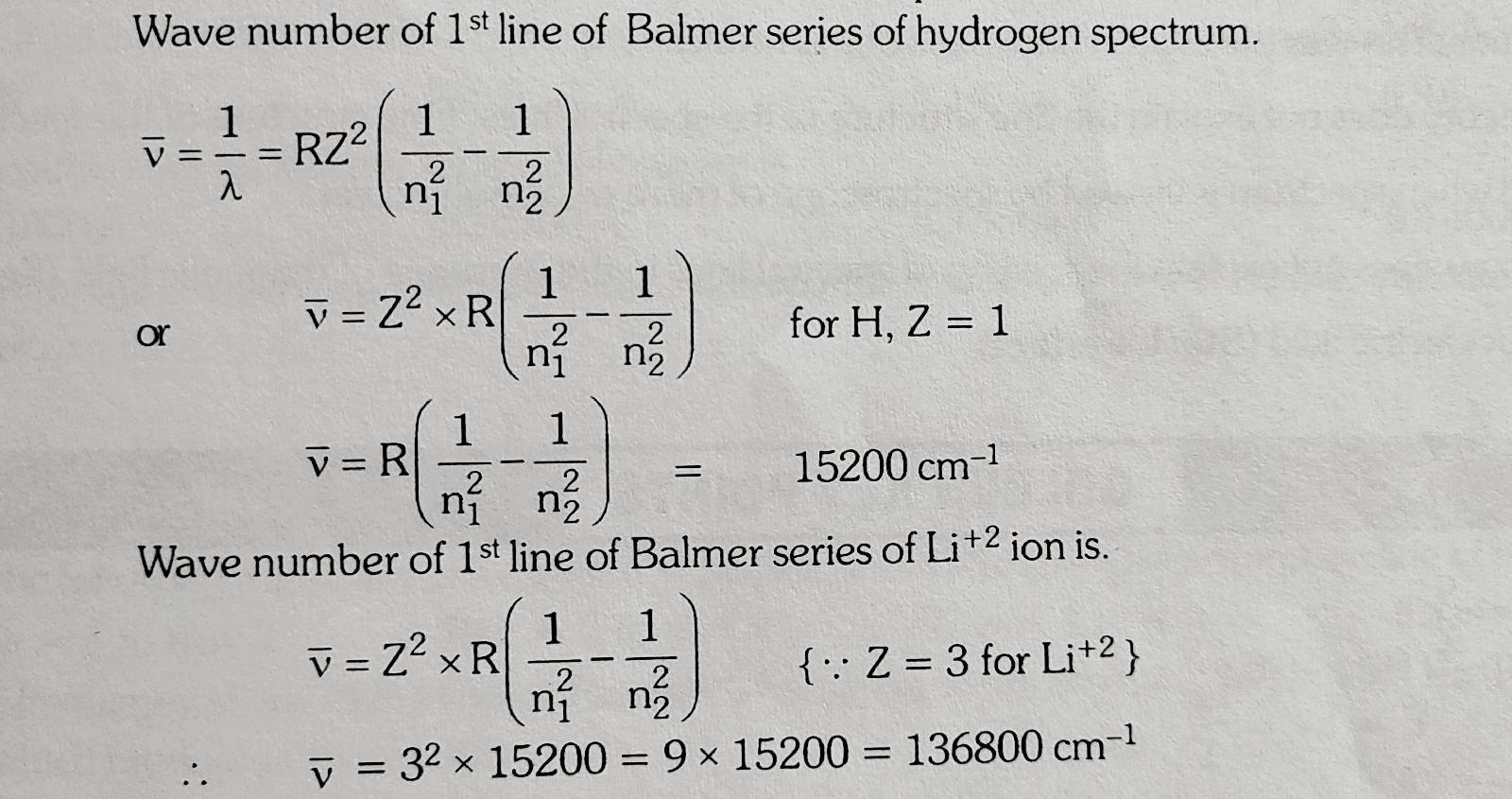Search This Blog
Showing posts with label Hydrogen spectrum. Show all posts
Showing posts with label Hydrogen spectrum. Show all posts
Friday, May 26, 2023
Thursday, May 25, 2023
A certain electronic transition from an excited state to Ground state of the Hydrogen atom in one or more steps gives rise to 5 lines in the ultra violet region of the spectrum.How many lines does this transition produce in the Infra red region of the spectrum?
Subscribe to:
Comments (Atom)