(2) The name is given
silicone because their empirical formula is analogous to that of ketones (R2CO).
(3) The Silicones are form by hydrolysis of
silicone tetra chloride (SiCl4) .we know that CCl4 do not
hydrolyses by water at room temperature While SiCl4 undergoes water Hydrolysis to corresponding OH group.
CCl4 +H2O¾® no hydrolysis
But super heated steam gives phosgene gas.
CCl4 +
H2O ¾®COCl2 +
2HCl
(4) SiCl4 under goes hydrolysis due to
presence vacant d orbital and gives Silicic acid followed by dehydration gives
3D Silicates (SiO2)
PREPARATION OF SILICONES:
It is two step process
Step: (1) Preparation of Organosilicon halides as silicone intermediates.
Step: (2) hydrolysis of Organosilicon halides followed by
condensation polymerisation.
Step: (1) [A] FROM FRIGNARD REAGENT:
Step: (1) [B] BY DIRECT HEATING PROCESS:
Note-The Yield of above reaction
is 50% R2MgCl2 and 50 % ( R3MgCl + RMgCl3),
now these can seperated by fractional distilation.
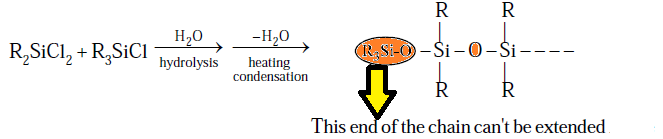
Step: (2) HDROLYSIS FOLLOWED BY CONDENSATION:
(1) RMgCl3 (R = Me or Ph): On hydrolysis and followed by condensation give 3D network
cross linked Silicoes It also provides the crosslinking among the chain making
the polymer more hard and hence controlling the proportion of RSiCl3
we can control the hardness of polymer.
(2) R2SiCl2 (R = Me or Ph): On
hydrolysis and followed by condensation
give linear as well as cyclic Silicones depending upon number of Silicon atomes.
Commercial silicon
polymers are usually methyl derivatives and to a lesser extent phenyl
derivatives. They are prepared by the hydrolysis of R2SiCl2 (R
= Me or Ph).
Note-Silicones may
have the cyclic structure also having 3, 4, 5 and 6 nos. of silicon atoms within
the ring. Alcohol analogue of silicon is known as silanol.
Note-Cyclic
Silicone have Sp3 oxygen and Silicon atoms so cyclic Silicones have following
properties .they are non planer ,polar and have Back Bonding and chair confermer.
(3) R3SiCl (R = Me or
Ph): On hydrolysis and followed by condensation so only dimmerisation take place due to
presence of single OH group.
Note- R3SiCl use in a certain proportion
we can control
the chain length of the polymer due to this reason R3SiCl is called
as chain stopping unit.
INERTNESS OF SILICONES: DUE TO..
(1) Silicones
are chemically inert due to back bonding
between oxygen and Silicone atoms
(2) High
bond energy of Si-C and Si-O bond also due to Back Bonding.
(3) Alkyl
group constitute hydrophobic part which act as water repellent hence
nucleophilic attack retarded .
USES OF
SILICONES:
(1) Silicones are
chemically inert, water repelling nature, heat resistance and having good
electrical insulating properties.
(2) Silicones are used
as sealants, greases, electrical insulators and for water proofing of fabrics, car
polish, shoe polish and masonry works in buildings
(3) Silicones can be
used as electrical insulator (due to inertness of Si-O-Si bonds)
(4) Silicones are used
as antifoaming agent in sewage disposal, beer making and in cooking oil used to
prepare potato chips.
(5) Silicones use as a
lubricant in the gear boxes



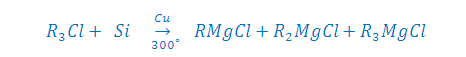



No comments:
Post a Comment