The zinc
sulphide crystals are composed of equal number of Zn+2 and S2- ions. The radii of the two
ions (Zn+2 = 74 pm and S-2 = 184 pm) led to the radius r+/r-
as 0.40 which suggests a tetrahedral arrangement.
The salient
features of this structure are as follows:
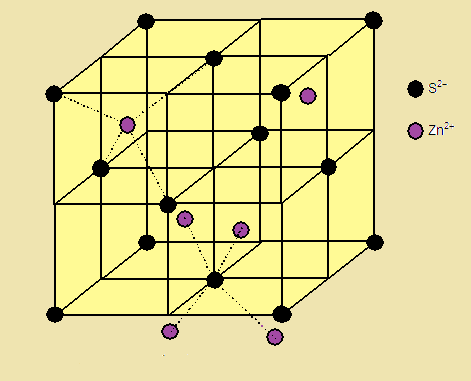
(1)The Sulphide ions are arranged in ccp
arrangement, i.e. sulphide ions are present at the corners and the centres of each face of the cube
(2) Zinc ions occupy tetrahedral hole. Only half (50 %) of the tetrahedral holes
are occupied by Zn+2 so that the formula of the zinc sulphide is ZnS
i.e. the stoichiometry of the compound is 1:1 (Only alternate tetrahedral holes are occupied
by Zn+2)
(3) Since the void is tetrahedral,
each zinc ion is surrounded by four sulphide ions and each sulphide ion is
surrounded tetrahedrally by four zinc ions. Thus zinc sulphide has [4:4] Coordination.
(4) For exact fitting of Zn+2
in the tetrahedral holes, formed by close packing of S-2 ions, the
ratio Zn+2/S-2 should be 0.225. Actually this ratio is
slightly large (0.40)
(5) There are four Zn+2
ions and four S-2 ions per unit cell as calculated below:
No.
of S-2 ions = 8(at corners)´1/8 + 6(at face centres)´1/2 = 4
No.
of Zn+2 ions = 4 (within the body)´1 = 4
(6) Density, Packing efficiency (PE) and Void % ;
Examples: Thus, the number of ZnS units per unit cell is equal to 4. Some more
examples of ionic solids having Zinc blende structures are CuCl, CuBr, CuI,
AgI, BeS (beryllium sulphide).
ILLUSTRATIVE EXAMPLE: If silver iodide crystallizes in a zinc
blende structure with I-
ions forming the lattice then calculate fraction of the tetrahedral voids
occupied by Ag+ ions.
SOLUTION: In AgI, if there are nI- ions, there will be nAg+
ions. As I- ions form the lattice, number of
tetrahedral voids = 2n. As there are nAg+ ions to occupy these
voids, therefore fraction of tetrahedral voids occupied by Ag+ ions
= n/2n = ½ = 50%.



No comments:
Post a Comment