When
roasting forms a liquid product, which makes separation easier, it is called smelting. Metal is extracted by heating calcined or
roasted ore with powdered coke in presence of a flux.
Consider,
for example, the smelting of zinc oxide:
The gaseous carbon monoxide separates from the liquid zinc, allowing the metal to be readily recovered. Other examples are
Slag:
In some
cases, a flux must be added to the mixture during smelting to help separate the
two materials. The flux is a material
that will react with the gangue to form a substance with a low melting
point. For example, oxides of silicon within gangue can be liquefied by
reaction with calcium carbonate according to the reaction:
The waste liquid
solution that forms from the flux and gangue is usually a silicate material
called a slag.
The liquid metal and the liquid slag have different densities and therefore
separate. Holes tapped at different heights into the side of the container
holding the liquid metal and slag allow the more dense liquid to flow out of
the lower tap holes and the less dense liquid to flow out of the higher tap
holes.
Principle of Slag formation:
The
principle of slag formation is essentially the following:
Nonmetal oxide (acidic) +
Metal oxide (basic) to form
a Fusible (easily melted) slag
Removal
of unwanted basic and acidic oxides: For example, FeO is the impurity in
extraction of Cu from copper pyrite.
Matte
also contains a very small amount of iron (II) sulphide.
To
remove unwanted acidic impurities like sand and P4O10,
smelting is done in the presence of limestone.
Properties of a Slag:
(1) Slag
is a fusible mass.
(2) It has
low melting point.
(3) It is
lighter than and immiscible with the molten metal. It is due to these
impurities that the slag floats as a separate layer on the molten metal and can
thus be easily separated from the metal. The layer of the slag on the molten
metal prevents the metal from being oxidised.


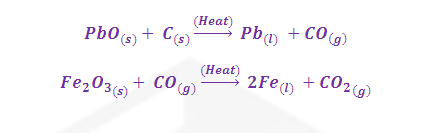



No comments:
Post a Comment