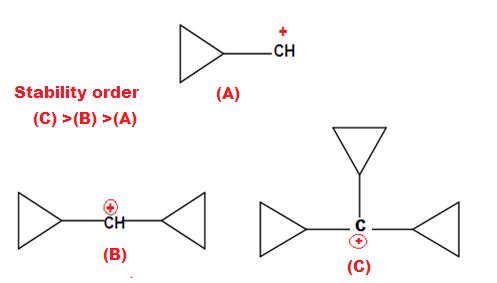
The exceptional stability of cyclopropane methyl cation can be explained by the concept of dancing resonance concept. The stability of additional cyclopropyl group , is result of more conjugation between the bent orbital of cyclopropyl ring and cationic carbon.
The most stable carbocation known till date in organic chemistry is explain by Dancing resonance.
Related Questions:


No comments:
Post a Comment