The Boranes unddergo
different type of chemical reactions like oxidation, pyrolysis, Nucleophilic
and electrophic and reactions with bases
such as OH- and NH3.
(1) Reaction
with Na: Diborane reacts with sodium amalgum to form an
addition product B2H6Na2.
(3) Thermal Stability: B2H6 stable
only at low temperature when heated 100 to 250 degree it changes into higher
hydrides and On heating to 700ºC diborane dissociates.
Note: - Formation of B2H2Cl4 shows that the 2H left in B2H2Cl4 are responsible for dimmer formation (Bridge bond). Diborane has only four replaceable hydrogen and with their replacement, the dimeric structure continuous to be as such. Remaining two hydrogen when they get displaced, the dimeric structure break indicating that these two hydrogen are act as bridging hydrogen.
(9) Reaction with Ammonia (NH3):
(1) Diborane react with excess NH3 at temperature to form (inorganic graphite) Boron nitride (BN) x.while when diborane and NH3 react in 1:2 ratios at low temperature give Inorganic Benzene (Borazole).
(2) Diborane
is electron-deficient molecule and hence it reacts with several molecules
having lone pair(s) of electron like CO, ether, amines, to
form complex compounds.
(3) B2H6 give symmetrical
cleavage with respect to only large
size and weak amines CO, H¯, N(CH3)3 ,
THF, PH3, PF3 , OEt3 OMe2 ,Pyridine,
Thiophene ,SMe2, Set3 etc.
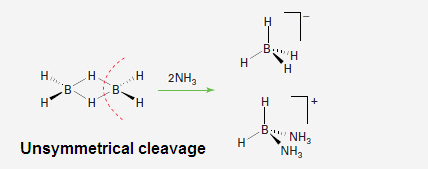
(4) In
the presence of small and strong base B2H6 undergo unsymmetrical
cleavage like NH3, H2N
(CH3), HN (CH3)2 etc
(10)
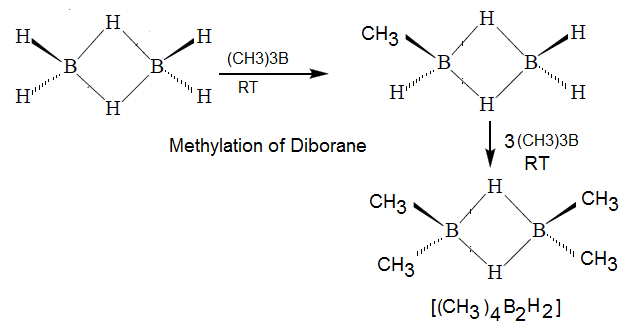
Methylation of Doborane:
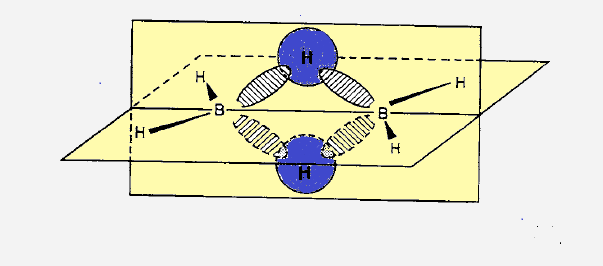
(1) In diborane two boron atoms and four terminal hydrogen atoms lie in one
plane. While two bridge hydogen atoms (encircled ) lie smmetrically above and
below the plane.
(2) Total valence electrons in B2H6 is 12(6 from boron
3x2) and 6 from six hydrogen atoms) and there are two B-H-B (3C-2e) bridge
bonds and four B-H (2C-2e) terminal bonds.
(3) Bond energy of B-H-B bond is 441 kjpermole
which greater than bond energy B-H bond (341Kjpermole)
hence methylation of diborane no more than four hydrogen.
(4) In above
reaction it is clearly reveal that none of the bridge hydrogen in B2H6
has been replaced by –CH3 .ie
in this reaction both the bridge bond remaine undissociate.
Structure of "Inorganic Benzene" /Borazole/Borazine:

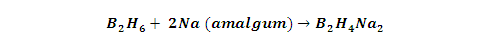
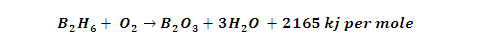



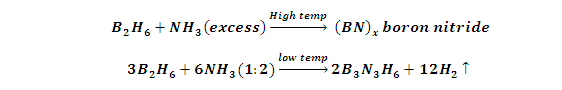


sd
ReplyDelete