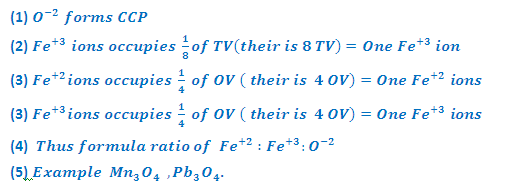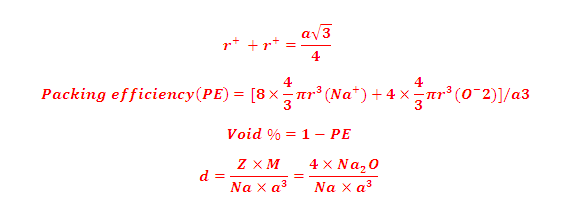The sodium chloride structure is composed of Na+ and Cl-
ions. The number of sodium ions is equal to that of Cl- ions. The
radii of Na+ and Cl- ions 95 pm and 181 pm giving the
radius ratio of 0.524
The radius ratio of 0.524 for NaCl suggest an octahedral void. Thus the salient features of this structure are as follows:
(1) Chloride ions (In a typical unit cell) are arranged in cubic close packing (ccp). In this arrangement, Cl- ions are present at the corners and at the centre of each face of the cube. This arrangement
is also regarded as face centred cubic arrangement (fcc).
(2) The sodium ions are present in all
the octahedral (Voids) holes.
(3) Since, the number of octahedral holes in ccp
structure is equal to the number of anions, every octahedral hole is occupied
by Na+ ions. So that the formula of sodium chloride is NaCl
i.e. Stoichiometry of NaCl is 1:1.
(4 ) Since there are six octahedral holes around each chloride ions, each
Cl- ion is surrounded by 6 Na+ ions. Similarly each Na+ ion
is surrounded by 6 Cl- ions. Therefore, the coordination number of
Cl- as well as of Na+ ions is six. This is called 6:6 coordination.
(A) Nearest neighbor of Na+
and Cl- ions is 6 (Six) at distance a/2.
(B) Next nearest Na+
and Cl- ions is 12 at distance a/root 2
(5) It should be noted that Na+ ions to exactly fit the
octahedral holes, the radius ratio of sodium and
chloride ions should be equal to 0.414. However, the actual radius ratio 0.524 exceeds
this value. Therefore to accommodate large Na+ ions, the
Cl- ions move apart slightly i.e. they do not touch each other and
form an expanded face centred lattice.
(6) The unit cell of sodium chloride has 4 sodium and 4 chloride ions
as calculated below
No of sodium ions = 12 (at edge centres) ´1/4 + 1 (at body centre)´1= 4
No of chloride ions = 8(at
corner)´1/8+6
(at face centres) ´1/2 = 4
Thus, the number of NaCl units per unit cell is 4.
(7) The edge length of the unit cell of NaCl type of crystal is 2(r+R) where
r = radii of Na+ ion and R is radii of Cl-
Thus, the distance between
Na+ and Cl- ions = a/2
(8) Density and packing efficiency of NaCl are as:
Examples of NaCl
type ionic salts:
Most of the halides of alkali metals, oxides
and sulphides of alkaline earth metals have this
type of structures.
(1) Group 1st Halides
ie NaI, KCl, RbI, RbF ( except Cs halides) .
(2) Group 2nd oxide MgO, CaO, BaO, SrO ( except BeO)
(3) Ammonium Halides ie NH4Cl
,NH4Br ,NH4I etc
(4) Silver Halides ie AgF, AgCl,
AgBr ,( except AgI)
(5) Other examples , TiO ,FeO,
NiO etc
Note: Ferrous oxide also has sodium chloride, types structure in which O-2
ions are arranged in ccp and Fe+2 ions occupy octahedral ions. However,
this oxide is always non – Stoichiometric and has the composition Fe0.95 O It can be explained on the assumption that some of the Fe+2
ion are replaced by 2/3rd as many Fe+3 ions in the
octahedral voids.