The compound having A2B formula
are compounds having anti fluorite structure :
Anti fluorite structure is having
arrangement of cations and anions opposite to the fluorite structure Li2O has
an anti fluorite structure.
(1) In the crystal structure of Li2O,
the O-2 ions constitute a cubic close packed lattice (fcc structure)
and the Li+ ions occupy all the tetrahedral voids
(2) Each oxide ion, O-2
ion is in contact with 8 Li+ ions and each Li+ ions
having contact with 4 oxide ion. Therefore, Li2O has 4:8
coordination
(3) Stoichiometric ratio of Na2O is 2:1
(4) radius ratio , Packing efficiency, density and void %:
(3) Stoichiometric ratio of Na2O is 2:1
(4) radius ratio , Packing efficiency, density and void %:
Other Examples:
– Na2O, K2O, K2S, Na2S, Rb2O,
Rb2S
Note:
Metals like Al, Ag, Au, Cu, Ni, and
Pt have ccp structure and Be, Mg, Co and Zn have a hcp structure. And Noble
gases (except He has a hcp structure) have ccp structure.


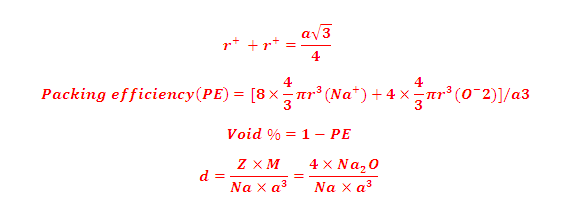
No comments:
Post a Comment