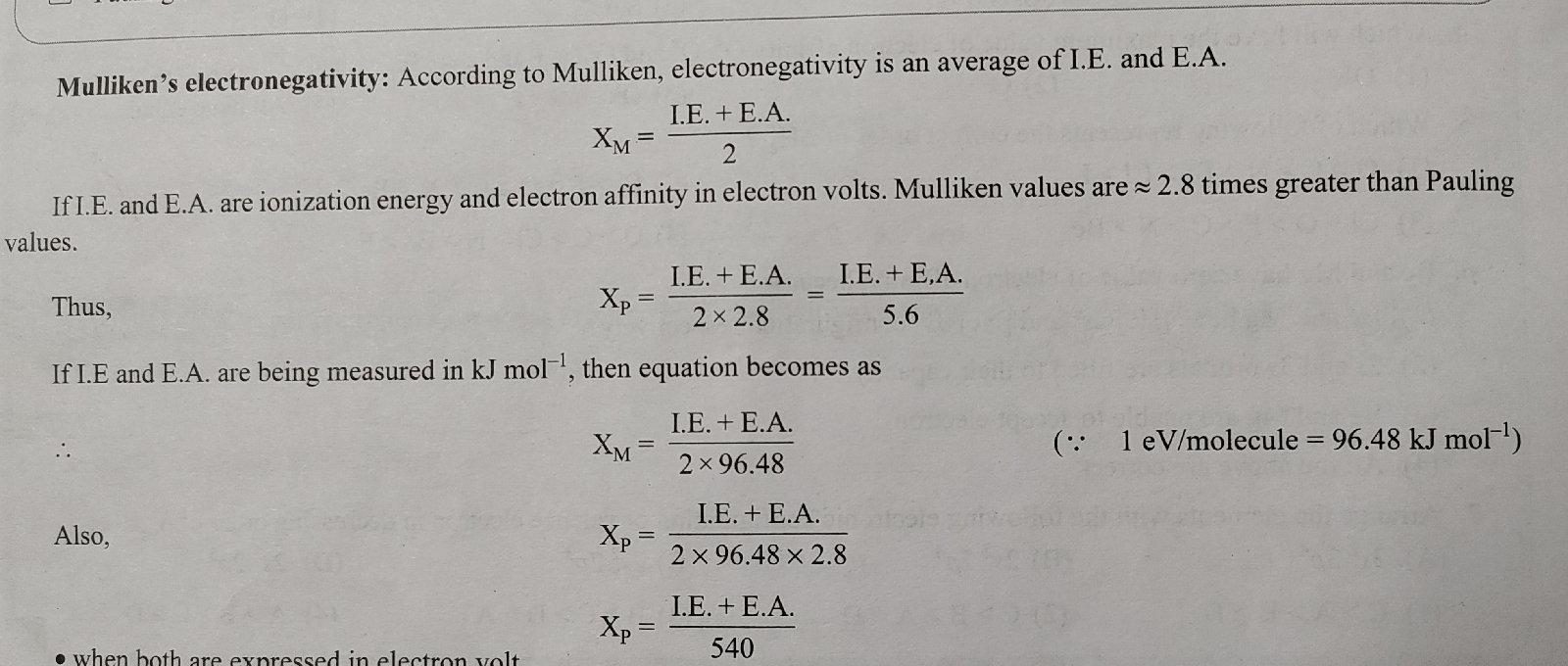Search This Blog
Showing posts with label PERIODIC TABLE. Show all posts
Showing posts with label PERIODIC TABLE. Show all posts
Sunday, May 25, 2025
Mulliken's Electronegativity:
Wednesday, May 21, 2025
Order of First Ionisation Enthalpy of Boron or 13th Group
B > Tl > Ga > Al > In ( IE1- Order)
Subscribe to:
Comments (Atom)