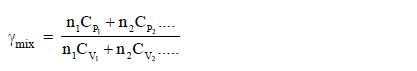Search This Blog
Showing posts with label THERMODYNAMICS:. Show all posts
Showing posts with label THERMODYNAMICS:. Show all posts
Sunday, December 22, 2024
The internal energy of one mole of a gas is
A sample of gas is compressed by an average pressure of 0.50 atmosphere so as to decrease its volume from 400 cm^3 to 200 cm3^.During the process 8.00 J of heat flows out to surroundings. Calculate the change in internal energy of the system.
500 gm ice at 0ºC is added in 2000 gm water at tºC. If the final temperature of system is 0ºC, then the value of 't' is (latent heat of fusion of ice =80 cal/gm and specific heat capacity of water= 1 cal/gm-ºC)
One mole of argon gas (assuming ideal behaviour) is expanded reversibly and adiabatically from 2.5 m^3 at 300K temperature to 5.0 m^3. Cv m for argon is 12.55 JK^–1mol^–1.Calculate the final temperature and pressure of the gas.
Wednesday, December 18, 2024
Heat, Heat Exchange and Heat capacity (C) :
Heat is defined as the energy that flow into or out of the system because of a difference in temperature between system and surrounding.
Note; Work is more organized way of energy transfer as compared to hear exchange.
IUPAC Sign convention of Heat:
Sign of heat will negative (-Ve) if heat is released by the system given by system.
While sign of heat will be positive (+Ve) if heat is given to the system.
(i) qV = n Cv dT (for constant volume process)
(ii) qp = n Cp dT (for constant pressure process)
(iii) (Cm)p – (Cm)v = R
(iv) Cv & Cp depends on temperature even for an ideal gas.( C = a + bT + cT2 .....)
(v) It is a path function
(vi) Cv, Cp are heat capacity of system and (Cm)v & (Cm)p are heat capacity of one mole system at constant volume and pressure respectively.
“Exchange of heat and work(P–V) in between system and surrounding always
occur through boundary of system”
Note: heat exchange can be measured with the help of Heat Capacity.
HEAT CAPACITY(C)
We know that usually on increasing in temperature is proportional to the heat transfer
q = coefficient x ∆T
The coefficient depend on the size, composition and nature of the system so we can also write it as
q= C∆T
where C is called Heat capacity
The heat capacity (C):
q=C∆T or
q=C∆T or
C= q/∆T unit- J/K
If ΔT=10=1K
Then C=qIt is equal to amount of heat needed to raise the temperature of the sample of any substance by one degree Celsius (or Kelvin).
heat Capacity depend on quantity, nature as well as physical state of the system. And the heat capacity is extensive. it may be made intensive as specific heat capacity
Specific Heat Capacity(Cs ):
q=Csm∆T or
Cs= q/m∆T
If ΔT=10=1K and m = 1g
Then Cs=q
It is equal to amount of heat required to raise the temperature of 1gm substance by one degree centigrade .it is intensive properties.Molar Heat Capacity(Cm ):
q=Cmn∆T or
q=Cmn∆T or
Cm= q/n∆T
If ΔT=10=1K and n=1mole
Then Cm=q
It is equal to amount of heat required to raise the temperature of 1 Mole substance by one degree centigradeEXAMPLE(1): The latent heat of fusion of ice at 0ºCis 80 cal/gm the amount of heat needed to convert 200 gm ice into water at 0ºC is ?
(A) 80 cal (B) 16000 J (C) 16000 cal (D) 1600 cal
SOLUTION: Ans (C) q = m.L = 200 × 80 = 16000 cal
EXAMPLE(2): Calculate the amount of heat required to raise the temperature of 50 gm water from 25ºC to 55ºC.Specific heat capacity of water = 4.2 J/ºC-gm.
(A) 126 J (B) 210 J (C) 6300 J (D) 1500 J
SOLUTION: Ans. (C)
q = m.s.ΔT = 50 × 4.2 × (55 – 25) = 6300 J
EXAMPLE (3): Five moles of a monatomic ideal gas is heated from 300K to 400K at constant pressure. the amount of heat absorbed is :
(A) 500 cal (B) 1500 cal (C) 2500 cal (D) 2500 J
SOLUTION: Ans. (C)
qp = CpΔT = n.Cp,m ΔT
= 5 × 5/2× (400 – 300) = 2500 cal
EXAMPLE (4): 2 moles of an ideal gas absorbs 720 cal heat when heated from 27ºC to 87ºC, at constant volume. 'ɤ' for the gas is :
(A) 1.5 (B) 1.4 (C) 1.6 (D) 1.33
SOLUTION :Ans. (D)
qv = n.Cv,m. ΔT
Cv,m =qv/nΔT
= 720/2x(87 – 27)
= 6 cal/K-mol
Now, r = 1 +R/Cv,m
= 1 + 2/6
= 1.33
EXAMPLE (5): 500 gm ice at 0ºC is added in 2000 gm water at tºC. If the final temperature of system is 0ºC, then the value of 't' is (latent heat of fusion of ice = 80 cal/gm and specific heat capacity of water=cal/gm-ºC)
(A) 20 (B) 40 (C) 10 (D) 2
SOLUTION: Ans. (A)
Heat lost by water = heat gained by ice
or, (m.s. ΔT)water = (m.L)ice
or, 2000 × 1 × (t – 0) = 500 × 800
Δ t = 20ºC
EXAMPLE(6): What is the heat in Joules required to raise the temperature of 25 grams of water from 0 °C to 100 °C? What is the heat in calories?
(Given: specific heat of water = 4.18 J/g·°C)
SOLUTION: Use the formula q = mcΔT
where
q = heat energy
m = mass
c = specific heat
ΔT = change in temperature
q = 25gx4.18 J/g·°x(100 °C - 0 °C)
q = 25gx4.18 J/g·°Cx(100 °C)
q = 10450 J
where
q = heat energy
m = mass
c = specific heat
ΔT = change in temperature
q = 25gx4.18 J/g·°x(100 °C - 0 °C)
q = 25gx4.18 J/g·°Cx(100 °C)
q = 10450 J
We know 1 Calorie=4.18 J
So 10450 J in Calorie = 10450/4.8=2500 calorie
FOR LARGE HEAT CHANGE :
Q= nCm (T2-T1)
Case- (2) Cm = f(T)
Cm = a + bT+ cT2 +……..
Case- (3) The theoretical value of Cvm and Cpm for Ideal gas can determined by using degree of freedom.
CHARACTERISTIC OF HEAT CAPACITY:
(1): The heat capacity Of any system should depend upon temperature because by increasing temperature of system different degree of freedom get excited.
(2): When temperature approaches zero then heat absorbed by the solid mainly converted into vibration potential energy of molecule resulting in very small increase in temperature, hence ‘C’ increases sharply with increase in temperature.
Normally C Directly proportional T3
(3): When the temperature at Melting point of solid ,then heat capacity becomes nearly constant for solid elements.
Molar heat capacity= 6.4 Cal/K mole
Or specific heat capacity x atomic weight=6.4 (Dulong and petite’s law)
(4): Exactly at melting point, the heat capacity become infinite as ΔT=0
(5): the heat capacity of liquid is greater than that of solid because of rotational degree of freedom also excited.
(6): In liquid heat capacity also depend upon temperature and also infinite at boiling point.
(7): the heat capacity of gas become less than liquid because all vibrational and rotational degree of freedom converted into translational degree of freedom.
(8): the heat capacity of gases depend upon their atomicity.
(9): If the heat capacity depends upon temperature.
(10); As heat (q) is path function, any substance may have infinite heat capacity.
Example for any substance.
Isothermal process = infinite
Adiabatic process = 0
Isobaric process = Cp
Isochoric process = Cv
Normally ,we use Cp and Cv value as characteristic of substance.
Degree of Freedom and Law of Equipartion of Energy:
It is equal to number of modes of energy transfer when a gaseous molecule undergoes collision. OR
It represent the number of independent modes to describe the molecular motion.
Total degree of freedom = 3N (Where N is Number of atom in molecule)
Total degree of freedom = 3N (Where N is Number of atom in molecule)
1- Translational degree of freedom is 3 (three) always for mono, di and t tri atomic molecule.
2- Rotational degree of freedom is zero for mono atomic gas, 2 (two) for diatomic molecules and 3 (three) for triatomic molecule
3-Vibrational degree of freedom is also zero for mono atomic gas and 1(one) diatomic gas molecule and for polyatomic gases Vibrational DOF is calculated individually.( fvib= 3N- ftrans+ frot)
Total degree of freedom:= ftrans + f rot + f vib & fvib= 3N- ftrans + f rot
Molecules N TDF
He 1 3
O2 2 6
CO2 3 9
NH3 4 12
PCl5 6 18
Case-1
Monoatomic Diatomic Triatomic (linear) Triatomic (Non linear)
f total =3 ftotal =6 ftotal =9 f total=9
f trans=3 ftrans =3 ftrans=3 ftrans=3
f rot =0 frot =2 frot = 2 frot =3
f vib =0 fvib =1 fvib = 4 fvib =3
q =n CmdT
qV=n CvmdT
(Cm)v=(dq/dT)v
By FLOT dq= dU+ dW
At constant volume dW=0 so dqv=dU
Hence (
LAW OF EQUIPARTIAL OF ENERGY :
Average energy associate with each molecule per degree of freedom is U= 1/2KT (where K is Boltz’s man constant.
Average energy associate with each molecule per degree of freedom is U= 1/2KT (where K is Boltz’s man constant.
Let degree of freedom is = f then U is U=1/2fkT
And U=1/2fkTNA per molecule we know kNA=R
U=1/2fRT and dU/dT=1/2fR
And dU/dT=Cv hence Cv=1/2fR
Cv=1/2ftransR +1/2frotR (Where Vib degree inactive in chemistry)
For ideal gas Cpm-Cvm=R and Gama= Cpm/Cvm
Adiabatic exponent :Adiabatic exponent (Gama) for a mixture of gas with different heat capacity is defined as :
where n1, n2 ........................ are moles of different gases
Example: Calculate change in internal energy of 10 gm of H2 ,when it's state is changed from(300K, 1Atm) to (500 K, 2Atm) ?
Solution: For ideal gas
Cv for H2 (diatomic) in low temperature range will be 5R as vibrational part is not included.
Extensive properties
The parameter whose value change on division known as extensive properties and these are depends on the mass (size, quantity) of the system.
- Volume
- Number of moles
- Mass
- Mole
- Free Energy (G)
- Entropy (S)
- Enthalpy (H)
- Internal energy (E&U)
- Heat capacity
- K.E.
- P.E.
- Gibbs free energy (G)
- Resistance
- Conductance
Extensive and Intensive properties:
1: Extensive properties are additive but intensive properties are non additive.
2: Ratio of two extensive property gives an intensive property.
3: An extensive property can be converted into intensive property by defining it per mole/per gram/ per liter
Subscribe to:
Comments (Atom)