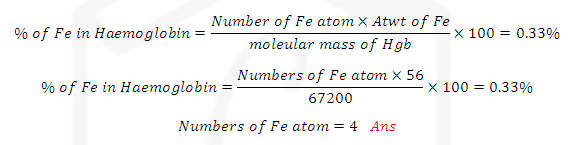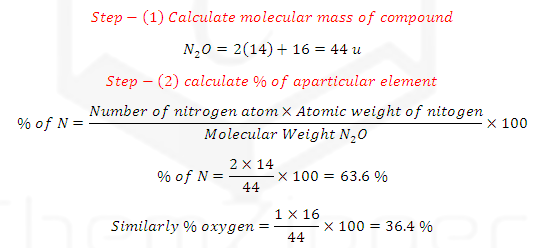SOLUTION:
Search This Blog
Showing posts with label PERCENTAGE COMPOSITION OF COMPOUNDS. Show all posts
Showing posts with label PERCENTAGE COMPOSITION OF COMPOUNDS. Show all posts
Saturday, September 26, 2020
Subscribe to:
Comments (Atom)