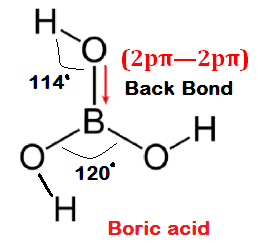
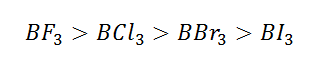Conditions for back bonding:
(1) Both of the atoms bonded with back Bonding are must be present in 2nd-2nd or 2nd-3rd period. 4rth period onward back bonding does not take place.
(2) One of
the atoms has lone pair (donor atom) and another (acceptor atom) have vacant
Orbital and direction of back Bonding depends upon vacant Orbital.
(3) The donor atom must have localized donatable electron pair and there should be inter electronic repulsion (smaller size). In general these are later half second period P - block elements
(F, O, N and C).
(4) The acceptor atom must have low energy empty orbital which
generally are np or nd orbitals. Small and similar size orbital’s favour overlap.
(5)
Back bonding is a weak pi bond thus only effective overlapping will be form
back bonding.
(6) Back bonding is found to be effective and considerable in following type of overlapping.
(i)
2p-2p
(ii) 2p-3p
(iii) 2p-3d
(7) The extent of overlapping order is [2p-2p> 2p-3d >2p-3p]
(8) dx2-y2 and dx2 Orbital’s does not participate in back Bonding.
Solution:
In case of trisilyl phosphine [P(SiH3)3] Phosphorous (P) being larger in atomic
size so it does not face much interelectronic repulsion and so, not much eager
to donate it’s loan pair electron and comfortable with it. So, it will not
donate to Si atom in this case. Thus, back bonding will not take place in trisilyl
phosphine [P(SiCH3)3].
But in the case of trisilyl amine [N(SiH3)3],
the nitrogen (N)
atom is
smaller in atomic size leading to high interelectronic repulsion, so it
wants ease by donating it’s loan pair electron to other atoms near to
it. And Si has vacant d-orbital, so needy for it. Since
both conditions are follow here, hence back
bonding will take place.