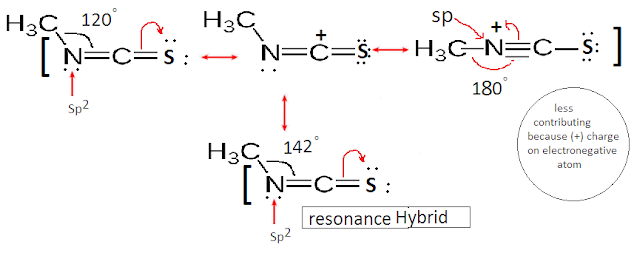
The hybridization of Nitrogen in Methyl isothiocyanate (H3CNCS) is Sp2. Thus bond angle (< C-N-C) is expected to be 120°. But it is slightly greater than 120° due to resonating structure. The resonating structure has N as Sp hybridized. Hence bond angle of the overall structure is found to be about 142°
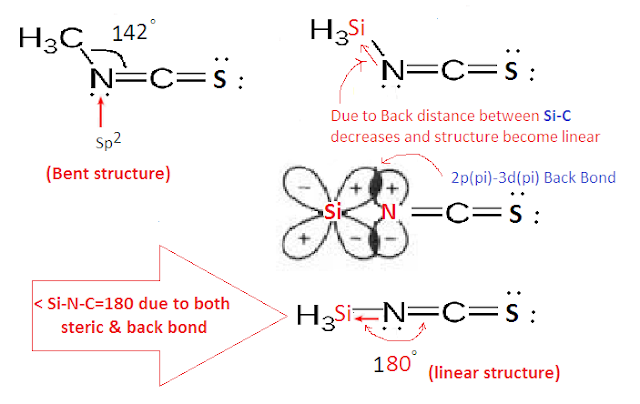
But in case Silyl isothiocyanate (SiH3NCO), the structure is found to be (sp hybridized)
and planar due to back bond between Si-N. In which the lone pair of electrons on N are donated to the vacant 3d orbitals of Si through back bonding (2pπ-3dπ back bond).
No comments:
Post a Comment