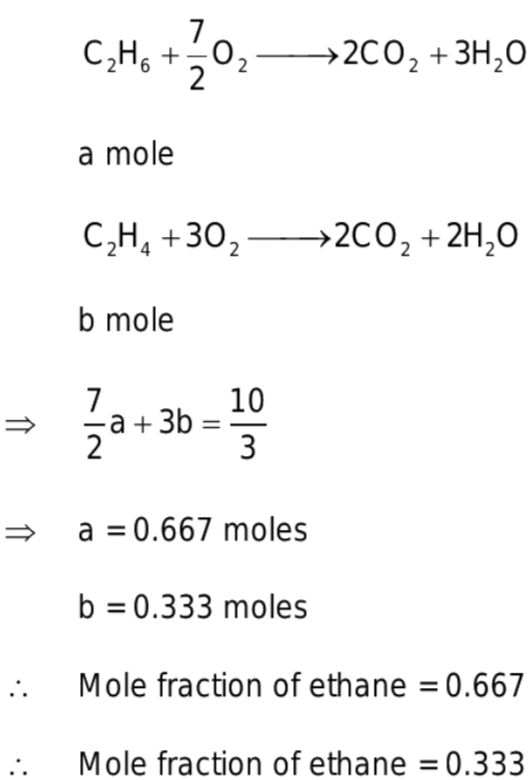Search This Blog
Showing posts with label EUDIOMETRY-VOLUME-VOLUME ANALYSIS OF GAS. Show all posts
Showing posts with label EUDIOMETRY-VOLUME-VOLUME ANALYSIS OF GAS. Show all posts
Saturday, May 27, 2023
A mixture of ethane (C2H6) and ethene (C2H4) occupies 40 litre at 1.00 atm and 400 K , the mixture reacts completely with 130 gm of O2 to produce CO2 and H2O . Assuming ideal gas behaviour , calculate the mole fraction of ethane (C2H6) and ethene (C2H4) in mixture. (R= 0.0821 atm L par mole par kelvin) (IIT 1915)
A mixture of ethane and ethene occupies 35.5 L at 1bar and 405K. The mixture completely reacts with 110 gm of O2 to produce CO2 and H2O. What was the composition of original mixture or what was themoles of ethane and ethene in the mixture (Given R= 0.0821 L atm K^-1mole^-1)
A mixture of ethane and ethene occupies 41L at 1atm and 500K. The mixture completely reacts with 10/3 mole of O2 to produce CO2 and H2O.The mole fractions of ethane and ethene in the mixture are respectively (Given R= 0.0821 L atm K^-1mole^-1)
25 gm of an unknown hydrocarbon upon burning produce 88 gm of CO2 and 9 gm of H2O. this unknown hydrocarbon contains.
(A ) 18g of carbon and 7g of hydrogen
(B) 20g of carbon and 5g of hydrogen
(C) 22g of carbon and 3g of hydrogen
A mixture of C 3 H 8 (g) & O 2 having total volume 100 ml in an Eudiometry tube is sparked & it is observed that a contraction of 45 ml is observed what can be the
C3H8(g)+5O2(g)→3CO2(g)+4H2O(l)
As par above equation 1 mole of porpane reacts with 5 moles of oxygen to form 3 moles of carbon dioxide.
We know that the number of moles is directly proportional to the volume of the gas.
Thus when 15 ml of propane reacts with 75 ml of oxygen, 45 ml of carbon dioxide will be obtained.
The contraction in the volume will be 15+75−45=45ml.
This is possible when the composition of the reacting mixture is either 15ml C3H8 and 85ml O2 or 25ml C3H8 and 75ml O2.
Hence, options A and B are the correct answers.
Friday, October 22, 2021
50 ml of dry ammonia gas was sparked for a long time in an eudiometer tube over mercury. After sparkling, the volume becomes 97 ml. After washing the gas with water and drying, the volume becomes 94 ml. This was mixed with 60.5 ml of oxygen and the mixture was burnt. After the completion of the combustion of H2 , the volume of residual gas was 48.75 ml. Derive the molecular formula of ammonia. (Given, N and H are only present in ammonia molecule).
Related Questions:
(1) 1 ml of gaseous aliphatic compound CnH3nOm is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(2) 1 ml of gaseous aliphatic compound CxHyOz is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(3) 50 ml of C3H8 is mixed with 300 ml of oxygen for complete combustion find out the final volume and volume concentration.
10 ml of gaseous hydrocarbon on combustion gives 40 ml. of CO2 (g) and 50 ml of H2O (vap). The hydrocarbon is
Related Questions:
(1) 1 ml of gaseous aliphatic compound CnH3nOm is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(2) 1 ml of gaseous aliphatic compound CxHyOz is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(3) 50 ml of C3H8 is mixed with 300 ml of oxygen for complete combustion find out the final volume and volume concentration.
Saturday, May 15, 2021
50 ml of C3H8 is mixed with 300 ml of oxygen for complete combustion find out the final volume and volume concentration.
Related Questions:
(1) 1 ml of gaseous aliphatic compound CnH3nOm is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
Sunday, April 19, 2020
1 ml of gaseous aliphatic compound CxHyOz is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
Contraction of volume is volume of gaseous mixture taken minus , volume of gaseous products obtained in a combustion reaction. In combustion of Hydrocarbon water obtained at room temperature is in liquid state hence it's volume ignored in calculation of volume contraction.
Related Questions:
(1) 1 ml of gaseous aliphatic compound CnH3nOm is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(2) 1 ml of gaseous aliphatic compound CxHyOz is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
(3) 50 ml of C3H8 is mixed with 300 ml of oxygen for complete combustion find out the final volume and volume concentration.
1 ml of gaseous aliphatic compound CnH3nOm is completely burnt in an excess of O2 and cooled to room temperature. The contraction in volume is..
Contraction of volume is volume of gaseous mixture taken minus , volume of gaseous products obtained in a combustion reaction. In combustion of Hydrocarbon water obtained at room temperature is in liquid state hence it's volume ignored in calculation of volume contraction.
Subscribe to:
Comments (Atom)