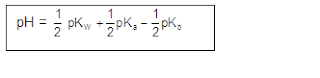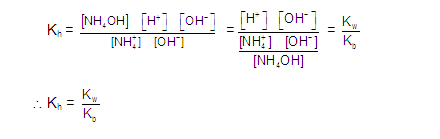Search This Blog
Sunday, September 10, 2023
Saturday, September 26, 2020
What are DOUBLE SALTS ?
Sunday, November 4, 2018
[5] POLYVALENT ION HYDROLYSIS:
For example Ca+2 , Fe+3 , Al+3 , Mn+2 , Cd+2 , Zn+3 etc
ILLUSTRATIVE EXAMPLE:
Calculate pH and concentration of all species in 0.1 M solution of FeCl3 given Fe(OH)3 have Kb1=10-3 , Kb2=10-7 and Kb3=10-12.
ILLUSTRATIVE EXAMPLE:
The following volume of 0.1M HCl Solution is added to the in20 ml 0.1M Na2CO3 Solution the pH of resulting solution in each case.
(H2CO3, Ka1= 4×10-6, Ka2= 5×10-11)
(1) 0.0 ml (No HCl added)
(2) 10 ml HCl is added
(3) 20 ml HCl is added
(4) 30 ml HCl is added
(5) 40 ml HCl is added
Saturday, November 3, 2018
Amphoteric salts hydrolysis:
Example
of amphoteric salts NaHS, NaHCO3, Na2HPO4,
NaH2PO4
(A) HCO3- act as conjugate acid as well as conjugate
base:
Both
reaction will support each other extent of hydrolysis and extent of
dissociation is same.
(B) Here H2PO4- and HPO4-2
are amphoteric anions. The pH of amphoteric salts anions is independent of
concentration of salts.
Here HPO4-2 is conjugate base of H2PO4- and H3PO4 is conjugate acid of H2PO4-Similarly PO4-3 is conjugate base of HPO4-2 and H2PO4-1 is conjugate acid of HPO4-2
When these salts are dissolved in water [H3O+]
concentration can be determined as;
ILLUSTRATIVE
EXAMPLE: Calculate pH
of solution of
(1) 100 ml
0.1M H3PO4 + 100 ml 0.1M NaOH.
(2) 100 ml
0.1M H3PO4 + 200 ml 0.1M NaOH.
(3) 100 ml
0.1M H3PO4 + 300 ml 0.1M NaOH.
(4) 100 ml
0.1M H3PO4 + 400 ml 0.1M NaOH.
Wednesday, October 31, 2018
[3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS:
[2] CATIONIC SALT HYDROLYSIS:
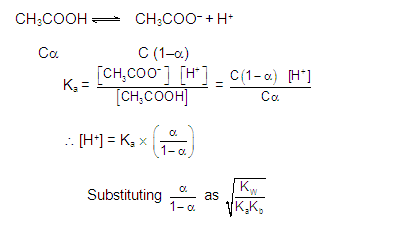
[1] ANIONIC SALT HYDROLYSIS:
(2) ALKALINE SALTS:(Salts of weak acids and strong bases)
(3) ACIDIC SALTS:(Salts of weak bases and strong acids)
(4) Salts of weak acids and weak bases