(2) CATIONIC HYDROLYSIS OR ACIDIC SALTS
HYDROLYSIS:
(Salt of a Weak Base and a
Strong Acid)
Let
the acid be HCl and the base be NH4OH. Therefore the salt would be
NH4Cl.
NH4Cl
completely dissociates into NH4+and Cl–
ions.
HCl
being a strong acid dissociates completely to give H+ ions and Cl–
ions.
In this hydrolysis, NH4OH and H+
are being produced. This implies that the solution is acidic
To calculate pH,
Multiplying
and dividing by OH– and rearranging,
Now,
substituting the concentrations,
ILLUSTRATIVE EXAMPLE (1):
ILLUSTRATIVE EXAMPLE (2):
ILLUSTRATIVE EXAMPLE (3):
ILLUSTRATIVE EXAMPLE (4):
ILLUSTRATIVE EXAMPLE (5):




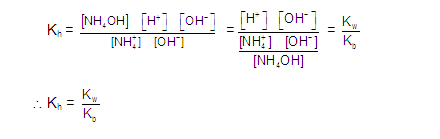

No comments:
Post a Comment