Take a salt (CH3COONH4) of the weak acid (CH3COOH) and the weak base (NH4OH) . and dissolve in water, therefore, the salt completely dissociates as given below.
The
ions get hydrolysed according to the reaction.
Such salts undergoes hydrolysis because ,the aqueous solution contains unionised acid as well as base molecules .
The nature of aqueous solution of such salt depends on the equilibrium constant for cationic or anionic hydrolysis.
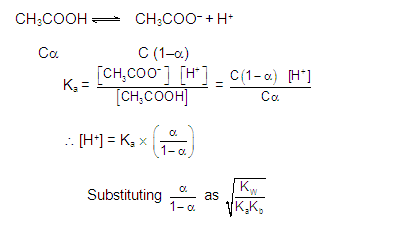
Multiplying and dividing by H+
& OH– and rearranging,
There is an important issue that needs clarification before we move on further. In this case,
we can see that both the ions (i.e., cation and anion) get hydrolyzed to produce a weak acid and a weak base (hence, we can’t predict whether the solution is acidic, basic or neutral). We have considered the degree of hydrolysis of both the ions to be the same. Now we present an explanation as to why this is incorrect and then state reasons for the validity of this assumption
n.
Actually
the hydrolysis reaction given earlier,
Now, we calculate the
pH of the solution as:
If the reaction for hydrolysis is in equilibrium then all the reversible processes occurring in water must be in equilibrium .
The H+ or OH- ions may be calculated from the dissociation constant of acid or base , here calculation of H+ from acid is given as below .
We know that at 25° Pkw of water is 14 .
Hence
pH = 7+ 1/2[Pka~pkb]
If Kh1<Kh2 then ka>kb. and pKa <pKb
as results Solution become acidic
If Kh1>Kh2 then ka<kB and pKa>pKb
as results solution become basic
ILLUSTRATIVE EXAMPLE (1): calculate the pH of 0.2 M NH4CN Solution. ( Given Ka HCN is 3x10-10 and kb NH4OH is 2.0x10-5)
(Ans-pH 9.5 )
ILLUSTRATIVE EXAMPLE (2):
Calculate the DOD and pH of 0.2M NaCN Solution (Given Ka of HCN is 2.0x10-10)
(Ans- DOD = √2×10-10 and pH =11.5)
ILLUSTRATIVE EXAMPLE (3):
Calculate the DOD (h) and pH of 0.2 M C6CH5NH3Cl Solution (Given Ka C6CH5NH3Cl is =4.0×10-8)
(Ans- DOD =√20×10-4 and pH is 6.6)
ILLUSTRATIVE EXAMPLE (4):
ILLUSTRATIVE EXAMPLE (5):







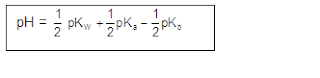
Thank you for your this blog post. It was very helpufull.
ReplyDelete