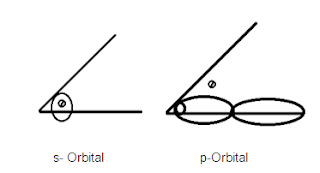
All the given molecules have same hybridization (sp3) and same central atom (P) the we can apply Bent's rule of hybridization. And according to Bent’s rule more electronegative atoms attach in those hybrid orbital’s which have less s-character or more electronegative atoms decreases s-character and increases p-character.
In
case of POF3 fluorine is more
electronegative hence s-character is
increase more and more in P=O bond as
compare to other given molecules so P=O bond is shortest in POF3 than others and also increase with decreasing
electronegativity. Hence P=O bond length
order is x1 < x2 < x3
< x4 and Bond strength order is reverse of bond length.
Related Questions: