Example
of amphoteric salts NaHS, NaHCO3, Na2HPO4,
NaH2PO4
(A) HCO3- act as conjugate acid as well as conjugate
base:
Both
reaction will support each other extent of hydrolysis and extent of
dissociation is same.
(B) Here H2PO4- and HPO4-2
are amphoteric anions. The pH of amphoteric salts anions is independent of
concentration of salts.
Here HPO4-2 is conjugate base of H2PO4- and H3PO4 is conjugate acid of H2PO4-Similarly PO4-3 is conjugate base of HPO4-2 and H2PO4-1 is conjugate acid of HPO4-2
When these salts are dissolved in water [H3O+]
concentration can be determined as;
ILLUSTRATIVE
EXAMPLE: Calculate pH
of solution of
(1) 100 ml
0.1M H3PO4 + 100 ml 0.1M NaOH.
(2) 100 ml
0.1M H3PO4 + 200 ml 0.1M NaOH.
(3) 100 ml
0.1M H3PO4 + 300 ml 0.1M NaOH.
(4) 100 ml
0.1M H3PO4 + 400 ml 0.1M NaOH.

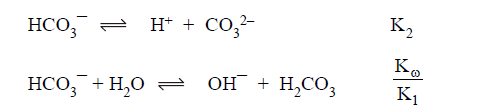



Pls elaborate how NaHs is an amphoteric salt.
ReplyDeleteNaHS is amphoteric salt it is ionised to give (NaHS--> Na+ + HS-) HS- ion which can donate H+ to form (S-2) sulphide ion as well as it can also accept H+ to form H2S hence NaHS is an amphoteric salt.
Delete