(A) CNC bond angle in H3CNCS is >120 and hybridization is closed to sp2
(B) Si-N-C bond angle is 180 in H3CNCS
(C) Both have Back Bonding
(D) Skeleton Si-N-C-S is linear but molecule are non planer.
SOLUTION:
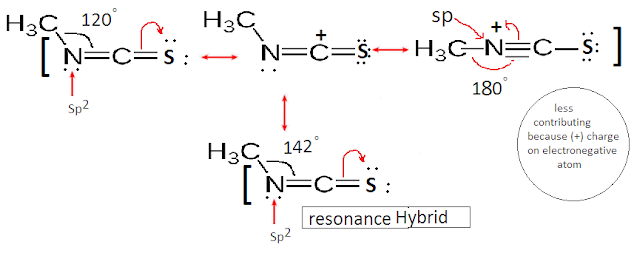
The hybridization of
Nitrogen in Methyl isothiocyanate (H3CNCS) is Sp2. Thus bond angle (< C-N-C) is expected to be 120°. But it
is slightly greater than 120° due to resonating structure.
The resonating structure has N as sp hybridized. Hence bond angle of the
overall structure is found to be about 142°
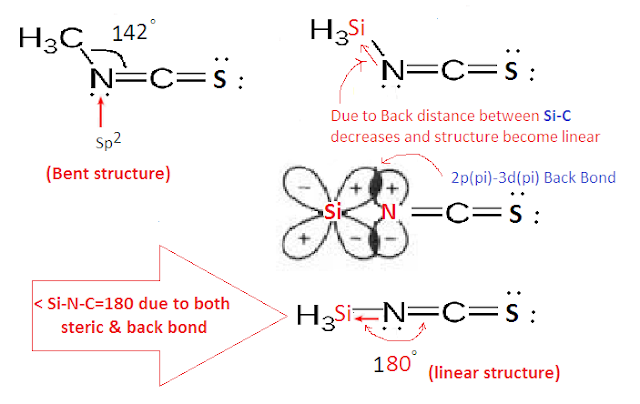
But in case Silyl isothiocyanate
(SiH3NCO), the structure is found to be (sp
hybridized) and planar due to back
bond between Si-N. In which the lone pair
of electrons on N are donated to the vacant 3d orbitals of Si through back
bonding (2pπ-3dπ back bond).
Hence Options (A, B, D) are
correct.

No comments:
Post a Comment