(1) Structure of FeO, Fe2O3, and Fe3O4 :
(1) FeO:
This oxide is non-stoichiometric and has a composition
FexO (Generally ‘x’ varying from0.92 to 0.97). The oxide ions form a cubic
close packing. The octahedral voids are occupied by Fe2+ but a small number of
Fe2+ is replaced by Fe3+ ions. Three Fe2+will
be replaced by two Fe3+ to maintain electrical neutrality but then what
we obtain is an iron-deficient crystal.
(2) Fe2O3:
If all Fe2+ are replaced by Fe3+, the ratio
between Fe:O will be 2 : 3 (since 3 Fe2+ are replaced by 2Fe3+)
and not 1 : 1. As such, we obtain Fe2O3.
(3) Fe3O4:
This is obtained by replacing exactly two
thirds of Fe2+ by Fe3+ (in FeO structure).The remaining Fe2+
ions and 50% of Fe3+ ions occupy the octahedral voids. The remaining
Fe3+ ions occupy tetrahedral voids. If in the structure of Fe3O4,
the Fe2+ ions are replaced by divalent cations such as Mg2+,
Zn2+, etc., the compounds obtained are called ferrites. In ferrites,
divalent cations occupy tetrahedral voids and trivalent cations occupy
octahedral voids. This structure is called spinel structure.
(2) NORMAL SPINEL (AB2O4 ) STRUCTURE:
Example of Spinel is a MgAl2O4.(
mineral) In it oxide ions (O-2) are arranged in ccp with Mg+2 ions occupying tetrahedral voids and Al+3
ions in a set of octahedral voids.
Many ferrites (such as ZnFe2O4)
also possess spinel structure. These are very important magnetic materials and
are used in telephone and memory loops in computers.
(3) INVERSE
SPINEL STRUCTURE (Fe3O4-Magnetite):
In
Fe3O4, Fe+2 and Fe+3 ions are present in the ratio 2:1.
it may be considered as having composition FeO.Fe2O3. In
Fe3O4 Oxide arranged in ccp. Fe+2 ions occupy
octahedral voids while Fe+3 ions are equally distributed between
octahedral and tetrahedral voids
MgFe2O4 also has structure similar
to magnetite. In this Mg+2 ions are present in place of Fe+2
ion in Fe3O4. Magnetite has inverse spinet structure.


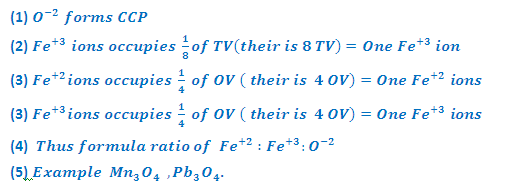
No comments:
Post a Comment