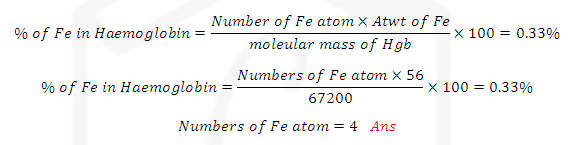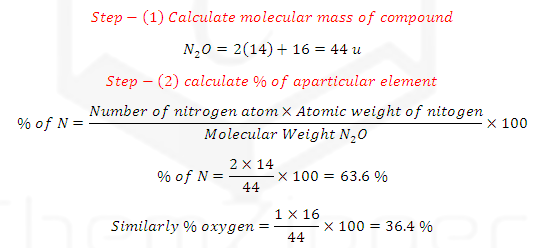SOLUTION:
Search This Blog
Showing posts with label SOME BASIC CONCEPT OF CHEMISTRY. Show all posts
Showing posts with label SOME BASIC CONCEPT OF CHEMISTRY. Show all posts
Saturday, September 26, 2020
What is the "Gram Molecular mass" ?
What is "Molecular mass ?
Molecular mass : Molecular mass is the number which indicates how many times the mass of one molecule of a substance is heavier in comparison to 1/12th part of the mass of one atom of C-12.
What is Gram atomic mass (GAM) ?
What is Atomic mass unit (amu) ?
The quantity [1/12 mass of an atom of C-12] is known as atomic mass unit. The actual mass of one atom of C-12 = 1.9924x10^-26 kg
What is "Atomic mass" ?
Atomic mass of an element can be defined as the number which indicates how many times the mass of one atom of the element is heavier in comparison to 1/12 th part of the mass of one atom of
Carbon-12.
What is "mole" ?
A mole is the amount of substance that contains as many species [Atoms, molecules ions or other particles] as there are atoms in exactly 12 gm of C-12
[1Mole= 6.022x10^23 Species]
Sunday, June 28, 2020
Saturday, June 6, 2020
40 gm metal oxide (MO) contain 4 gm of Oxygen.Calculate the equivalent wt of a metal.
40 gm metal oxide contains 4 gm of Oxygen mean it has 36 gm of metal.
Then Equivalent wt of metal is
E_m = (Wt of metal/Wt of Oxygen)×8
E_m = (36gm /4gm)×8
E_m = 72 gm equivalent
A metal oxide contains 60% metal by mass. The equivalent weight of metal is ..?
Metal Oxide contains 60% metal by mass means 100g of this oxide has 60 g metal and 40g oxygen.
Equivalent Wt of Metal = (Wt of metal/Wt of Oxygen)×8
E_m = 60×8/40 = 12gm equivalent
Monday, April 20, 2020
Subscribe to:
Posts (Atom)