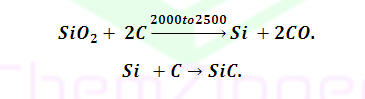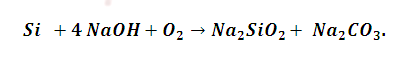Silicon
carbide (SiC) is a compound of silicon and carbon. It is
extremely rare on Earth in mineral form (moissanite) and it has semiconductor
properties. It has a bluish-black appearance. It has a large number of
crystalline forms.
Preparation: When Silica treated with carbon
at around 2000℃ to 2500℃ give carbide
Properties:(1)
SiC also known as carborundum which is very hard .(2)
Silicon carbide is inert and not attack by even HF.
(3) Silicon carbide when treated with NaOH give silicates and carbonate .
(3) Silicon carbide when treated with NaOH give silicates and carbonate .
(4) Silicon carbide is covalent
carbide.
(5) Silicon carbide has
a high sublimation temperature and is a good heat conductor. It's expansion
with increase in temperature is low. Also, it has high electric field breakdown
strength.
(6) Silicon carbide exhibits
the phenomenon of polymorphism, and hence has a large number of crystalline
forms. Alpha
silicon carbide is the most common polymorph and has a hexagonal
crystal structure, while Beta Silicon Carbide has zinc blende structure.