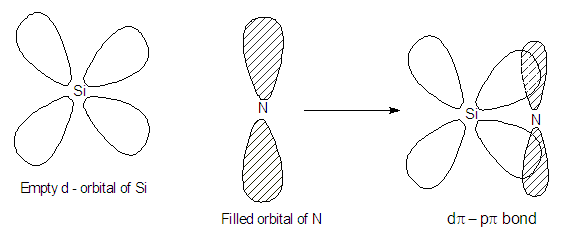
In N(SiH3)3, N attains sp2
hydridisation and the lone pair of N is involved in dpi-ppi
back bonding by getting itself
delocalized on to empty 3d – orbitals of silicon.
But in N(CH3)3, N is sp3
hybridised in which three of the hybrid orbitals are used in forming
s - bonds with NH3 groups, while the
lone pair is present in the fourth hybrid orbital. Thus the molecule is
pyramidal.
For More deatils click here Back Bond theory: