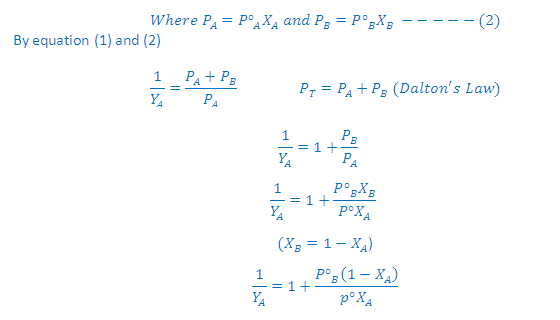According to Dalton's law of partial pressures, the total pressure PT is given by:
Partial pressure of the gas = Total pressure x Mole fraction
Where YA and YB is the mole fraction of the component A and B in gas phase respectively
According to Raoult's law:
On rearrangement of this equation we get a straight line equation:
Illustrative Examples: