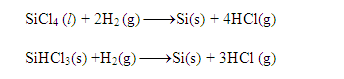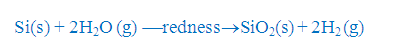(1) SILICON (Si):
(1) The Silicon was discovered by Berzelius in 1824.
(1) The Silicon was discovered by Berzelius in 1824.
(2) The silicon name is
taken from Latin silver which means “flint”.
(3) Silicon is the
second most abundant (27.2%) element after oxygen (45.5%) in the earth's crust.
(4) Silicon is a crystalline semi – metal or metalloid. One of its
forms is shiny, grey and very brittle. In another allotropic form silicon is a
brown amorphous powder most familiar in “dirty” beach sand.
(5) Silicon does not
occur free in nature but in the combined state, it occurs widely in form of silica (SiO2) and silicates (SiO4 -4) .
(6) All mineral rocks, clays
and soils are built of silicates of magnesium, aluminium, potassium or iron.
(7)
Aluminium silicate is however the most common constituent of rocks and clays.
(8)
the most common compound of silicon is Silica and Silica (SiO2) is
found in the Free State in sand, flint and quartz and
in the combined state as silicates like
(i) Feldspar K2O.Al2O3.
6SiO2
(ii) Kaolinite Al2O3.
2SiO2. 2H2O
(iii) Asbestos CaO. 3MgO. 4SiO2
(8) Silica (SiO2)
is the most abundant chemical compound in the earth’s crust.
PREPARATION SILICON:
(i) From silica (sand): Elemental silicon is obtained
by the reduction of silica (SiO2) with high purity coke in an
electric furnace
(ii) From silicon tetrachloride (SiCl4) or
silicon chloroform (SiHCl3): Silicon of very high purity required for making
semiconductors is obtained by reduction of highly purified silicon tetrachloride
or silicon chloroform with dihydrogen followed by purification by zone refining.
PHYSICAL
PROPERTIES:
(i) Elemental silicon is very hard
having diamond like structure.
(ii) It has shining luster with a melting
point of 1793K and boiling point of about 3550K.
(iii) Silicon exists in three
isotopes, i.e. 28Si14, 29Si14 and 30Si14
but 28Si14 is the most common isotope.
CHEMICAL PROPERTIES:
Silicon is particularly unreactive at
room temperature towards most of the elements except fluorine.
Some important chemical reactions of
silicon are discussed below.
(i) Action of air: Silicon reacts with
oxygen of air at 1173K to form silicon dioxide and with nitrogen of air at
1673K to form silicon nitride,.
(ii) Action of steam:
It
is slowly attacked by steam when heated to redness liberating hydrogen gas.
(iii) Reaction with halogens: It burns spontaneously
in fluorine gas at room temperature to form silicon tetra fluoride (SiF4).
However,
with other halogens, it combines at high temperatures forming tetra halides.
(iv) Reaction with carbon: Silicon combines with
carbon at 2500 °C forming silicon carbide (SiC) known as carborundum. Carborundum is an extremely hard
substance next only to diamond. It is mainly used as an abrasive and as a
refractory material.
USES OF
SILICON:
(i) Silicon is added to steel as such
or more usually in form of ferro silicon (an alloy of Fe and Si) to make it acid-resistant.
(ii) High purity silicon is used as
semiconductors in electronic devices such as transistors.
(iii) It is used in the preparation of
alloys such as silicon-bronze, magnesium silicon.
(2) SILICA (SiO2):
(2) SILICA (SiO2):
Silicon is unable to form pp -
pp bond with oxygen atom due to its relatively
large size. Thus it satisfies its all four valency with four oxygen atoms and
constitutes three - dimensional network. In this
structure each oxygen atom is shared by two silicon atoms. Three
crystalline modification of SiO2 are quartz, cristobalite
and tridymite of which quartz and cristobalite
are important.
Quartz (rock crystal) is the purest form of silica. It is used in
preparation of costly glasses and lenses. It is also used as piezoelectric material (crystal oscillators and
transducers).
Several
amorphous forms of silica such as silica gel and fumed silica are known. Silica
gel in made by acidification of sodium silicate and when dehydrates, is
extensively used as a drying agent in chromatographic and catalyst support.
Silicon
carbide (SiC) is a compound of silicon and carbon. It is
extremely rare on Earth in mineral form (moissanite) and it has semiconductor
properties. It has a bluish-black appearance. It has a large number of
crystalline forms.Silicon Carbide (SiC): Carborundum:
(1) Silicates is the
general term applied for the solids with silicon – oxygen bonds.
(2) Silicates are
regarded as the salts of silicic acid, H4SiO4.All the
silicates are comprised of SiO4 units.
(3)
The Silicates units have a tetrahedral structure formed as a result of sp3 hybridization.
Silicon atom has its complete octet but each oxygen atom is still short of one
electron to complete its octet. They can complete their octet by taking up 4 electrons
from a metal, getting converted to an anion [SiO4]-4
(4)
All the solids Silicates contain silicate ion (SiO4)4- as
the basic structural unit.
(5)The
silicate ion is tetrahedral in structure and when the one or more oxygen atoms
between such tetrahedrons, a complex structure arise.
(6) All
the silicates are non planer.
CLASSIFACATION OF SILICATES:
CLASSIFACATION OF SILICATES:
The
silicates may be classified in to following groups chain
silicates, ring silicates, cyclic silicates, sheet
silicates, three – dimensional silicates
depends on the way in which the (SiO4)4-
tetrahedral units are linked together
(1)
Ortho silicates or Neso Silicates
(2)
Pyro silicates or Sorosilicates or Disisilicates
(3)
Meta silicates (A) Cyclic meta silicates (B) Linear chain Meta Silicates
(4)
Double chain Silicates or Amphiboles
(5)
Sheet Silicates or phyllo silicates
(1) Silicones are organosilicon
polymeric compounds containing repeated R2SiO units and (-Si-O-Si-)
linkage.
(2) The name is given
silicone because their empirical formula is analogous to that of ketones (R2CO).
(3) The Silicones are form by hydrolysis of
silicone tetra chloride (SiCl4)
.
USES OF SILICONES:
.
USES OF SILICONES:
(1) Silicones are
chemically inert, water repelling nature, heat resistance and having good electrical
insulating properties.
(2) Silicones are used
as sealants, greases, electrical insulators and for water proofing of fabrics
SILICONES: (R2SiO WITH -Si-O-Si- LINKAGE):
SILICONES: (R2SiO WITH -Si-O-Si- LINKAGE):