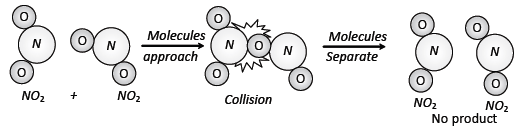
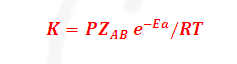(1) The basic requirement for a reaction to
occur is that the reacting species must collide with one another. This is the
basis of collision theory for
reactions.
(2) The number of collisions that takes
place per second per unit volume of the reaction mixture is known as collision frequency (Z).
(3) Every collision does not bring a
chemical change. The collisions that actually produce the product are effective collisions.
The effective collisions, which bring chemical change, are few in comparison to
the total number of collisions. The collisions that do not form a product are ineffective elastic
collisions, i.e., molecules just collide and disperse in
different directions with different velocities.
(4) For a collision to be effective, the
following two barriers are to be cleared.
(A) Energy barrier: "The
minimum amount of energy which the colliding molecules must possess as to make
the chemical reaction to occur is known as threshold
energy".
(i) In the graph 'E' corresponds to minimum
or threshold energy for effective collision.
(ii) There is an energy barrier for each
reaction. The reacting species must be provided with sufficient energy to cross
the energy barrier
(B) Orientation barrier: The
colliding molecules should also have proper orientation so that the
Old bonds
may break and new bonds are formed.
During
this reaction, the products are formed only when the colliding molecules have
proper
Orientation
at the time of collisions. These are called effective collisions.
(a) Properly oriented collisions form products
(b) Collisions not properly oriented
(5) Thus, the main points of collision
theory are as follows,
(i) For a reaction to occur there must be
collisions between the reacting species.
(ii)Only a certain fraction of the total
number of collisions is effective in forming the products.
(iii) For effective
collisions, the molecules should possess sufficient energy as well as
orientation.
(6) The fraction of effective
collisions, under ordinary conditions may vary from nearly zero to about one
for ordinary reactions. Thus, the rate of reaction is proportional to:
(i) The number of collisions per unit
volume per second (Collision frequency, Z) between the
reacting
species
(ii) The fraction of effective collisions
(Properly oriented and possessing sufficient energy), f
Where f is
fraction of effective collision and Z is the collision frequency.
(7) The physical meaning of the
activation energy is that it is the minimum relative kinetic energy which the
reactant molecules must possess for changing into the products molecules during
their collision. This means that the fraction of successful collision is equal
to e-Ea /RT called Boltzmann
factor.
(8) It may be noted that besides the
requirement of sufficient energy, the molecules must be
Properly
oriented in space also for a collision to be successful. Thus, if ZAB
is the collision frequency, P is the orientation factor (Steric factor) then
If we compare this equation with Arrhenius equation
We know that pre-exponential form 'A' in Arrhenius equation is, A= PZAB