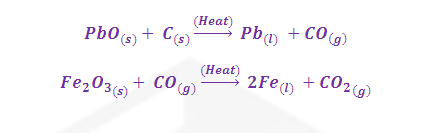The
ore is heated strongly below its melting point
in presence of excess of air which removes impurities of non – metals at their
volatile oxides.
(1) Roasting is exothermic process once started
it does not required additional heating.
(2) Roasting employed for Sulphide ores.
(3) Chemical Conversion of ore takes place.
(4) Roasting is carried out in Reverbatory furnace
The
process of roasting required the following:
(1) Conversion of sulphide ores to their respective
oxides.
(2) Conversion of sulphide ores to their
Sulphate.
Note: Some time roasting may not bring about
complete oxidation
(3) Roasting at high
temperature: the sulphide
ore of some of the metal like Cu, Pb, Hg, Sb, etc when heated strongly in the
free supply of air or O2 are reduced directly to the metal rather
than to the metallic oxide for example.
The
reduction of the sulphide ore directly into metal by heating it in air or O2
is called Self reduction, Auto reduction, Air reduction etc and the SO2 produced is utilised for manufacturing of Sulphuric acid.
Consequence of Roasting:
(1) Sulphide Ore is converted into oxide/sulphate
which may be further decomposed into metal oxide, example Sulphur dioxide
(2) Organic Matter is burnt away.
(3) Impurities of sulphure, phosphorous, arsenic
and antimony are oxidised into the respective volatile oxide.
(4) When concentrated tine Stone ore (SnO2)
is heated strongly in a free supply of air (roasting), the impurities of CuS
and FeS present in the ore are converted into CuSO4 and FeSO4
respectively.