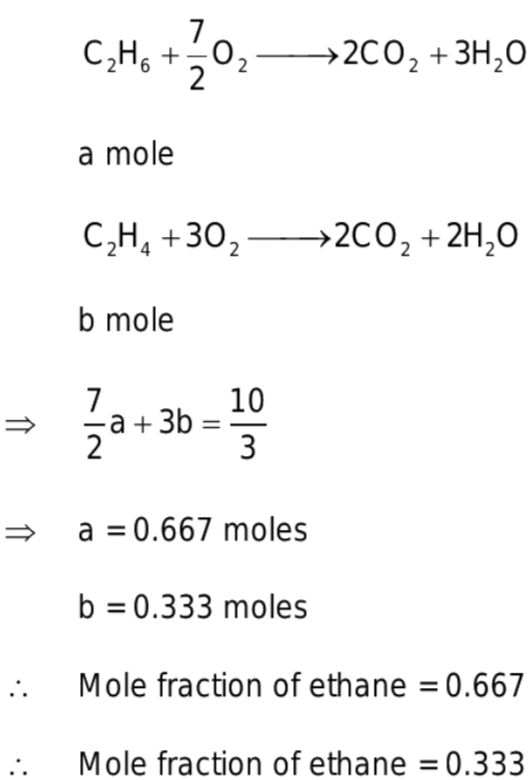Search This Blog
Saturday, May 27, 2023
A mixture of ethane and ethene occupies 41L at 1atm and 500K. The mixture completely reacts with 10/3 mole of O2 to produce CO2 and H2O.The mole fractions of ethane and ethene in the mixture are respectively (Given R= 0.0821 L atm K^-1mole^-1)
A solution contains Na 2 CO 3 and NaHCO 3 . 10 mL of the solution required 2.5 mL of 0.1 M H 2 SO 4 for neutralisation using phenolphthalein as indicator. Methyl orange is then added when a further 2.5 mL of 0.2 M H 2 SO 4 was required. Calculate the amount of Na 2 CO 3 and NaHCO 3 in one litre of the solution.
100mL of a water sample contains 0.81g of calcium bicarbonate and 0.73 of magnesium bicarbonate. The hardness of this water sample expressed in terms of equivalents of CaCO 3 is:(molar mass of calcium bicarbonate is 162g mol^−1 and magnesium bicarbonate is 146 gmol −1 )
(A) 1000 ppm
(B) 10000 ppm
(C) 100 ppm
(D) 5000 ppm
Solution :
neq.CaCO3=neqCa(HCO3)2+neqMg(HCO3)2
or
(W÷100) ×2 = (0.81÷ 162)×2 + (0.73÷146)×2
∴w=1.0
∴ Hardness=(1.0÷100) ×10^6=10000ppm
Hence correct option is (B) 10000ppm
Subscribe to:
Posts (Atom)