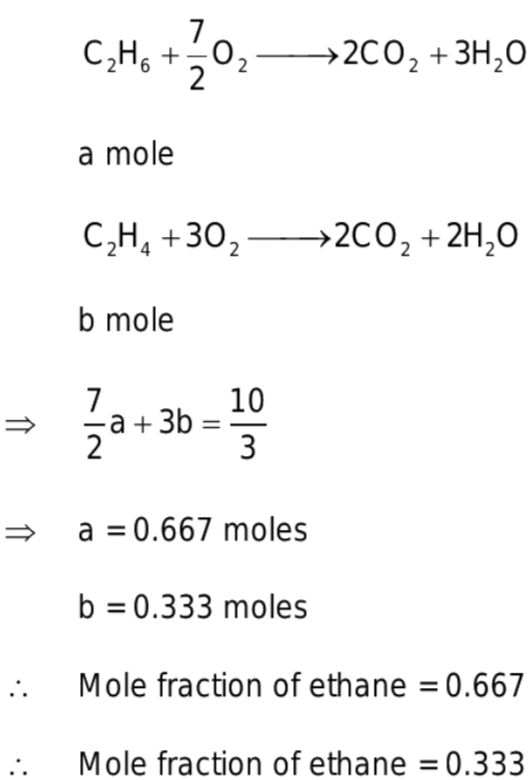Search This Blog
Saturday, May 27, 2023
A mixture of ethane (C2H6) and ethene (C2H4) occupies 40 litre at 1.00 atm and 400 K , the mixture reacts completely with 130 gm of O2 to produce CO2 and H2O . Assuming ideal gas behaviour , calculate the mole fraction of ethane (C2H6) and ethene (C2H4) in mixture. (R= 0.0821 atm L par mole par kelvin) (IIT 1915)
A mixture of ethane and ethene occupies 35.5 L at 1bar and 405K. The mixture completely reacts with 110 gm of O2 to produce CO2 and H2O. What was the composition of original mixture or what was themoles of ethane and ethene in the mixture (Given R= 0.0821 L atm K^-1mole^-1)
A mixture of ethane and ethene occupies 41L at 1atm and 500K. The mixture completely reacts with 10/3 mole of O2 to produce CO2 and H2O.The mole fractions of ethane and ethene in the mixture are respectively (Given R= 0.0821 L atm K^-1mole^-1)
Subscribe to:
Comments (Atom)