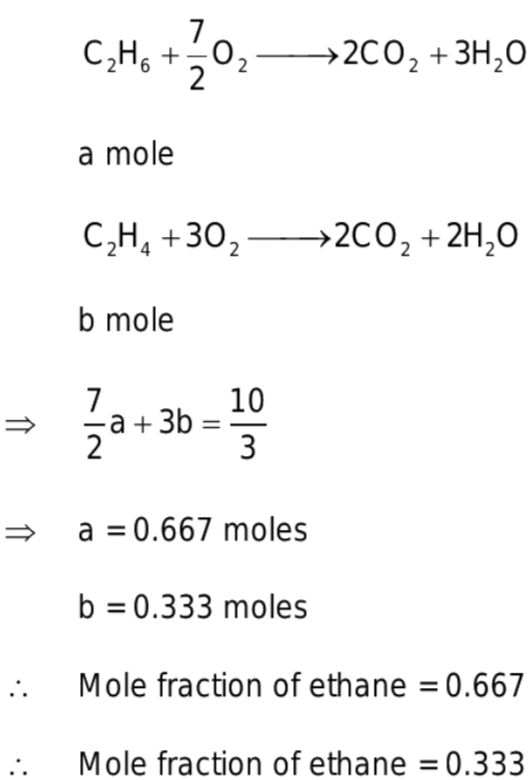Search This Blog
Saturday, May 27, 2023
A mixture of ethane and ethene occupies 35.5 L at 1bar and 405K. The mixture completely reacts with 110 gm of O2 to produce CO2 and H2O. What was the composition of original mixture or what was themoles of ethane and ethene in the mixture (Given R= 0.0821 L atm K^-1mole^-1)
A mixture of ethane and ethene occupies 41L at 1atm and 500K. The mixture completely reacts with 10/3 mole of O2 to produce CO2 and H2O.The mole fractions of ethane and ethene in the mixture are respectively (Given R= 0.0821 L atm K^-1mole^-1)
A solution contains Na 2 CO 3 and NaHCO 3 . 10 mL of the solution required 2.5 mL of 0.1 M H 2 SO 4 for neutralisation using phenolphthalein as indicator. Methyl orange is then added when a further 2.5 mL of 0.2 M H 2 SO 4 was required. Calculate the amount of Na 2 CO 3 and NaHCO 3 in one litre of the solution.
100mL of a water sample contains 0.81g of calcium bicarbonate and 0.73 of magnesium bicarbonate. The hardness of this water sample expressed in terms of equivalents of CaCO 3 is:(molar mass of calcium bicarbonate is 162g mol^−1 and magnesium bicarbonate is 146 gmol −1 )
(A) 1000 ppm
(B) 10000 ppm
(C) 100 ppm
(D) 5000 ppm
Solution :
neq.CaCO3=neqCa(HCO3)2+neqMg(HCO3)2
or
(W÷100) ×2 = (0.81÷ 162)×2 + (0.73÷146)×2
∴w=1.0
∴ Hardness=(1.0÷100) ×10^6=10000ppm
Hence correct option is (B) 10000ppm
25 gm of an unknown hydrocarbon upon burning produce 88 gm of CO2 and 9 gm of H2O. this unknown hydrocarbon contains.
(A ) 18g of carbon and 7g of hydrogen
(B) 20g of carbon and 5g of hydrogen
(C) 22g of carbon and 3g of hydrogen
Subscribe to:
Posts (Atom)