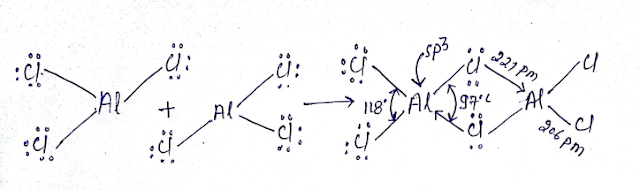
NAMING OF 1st GROUP: The group 1st containing
Li, Na, K, Rb, Cs & Fr , Francium is radioactive and has a very short life ( t1/2=
21 minutes ), therefore
very little is known about it. They are commonly called alkali metals because form hydroxide when react with water.
RELATIVE ABUNDANCE: Na
and K are 6th and 7th most abundance elements in earth
crust respectively and both Na, K constitutes 4% of total earth crust.
Na >
K > Rb > Li > Cs
STRUCTURE OF ALKALI METALS: At normal temperature all
the alkali metals are adopt BBC (Body Centred
cubic) type lattice with coordination number (8) but at low temperature Li adopt HCP with
coordination number (12).
ELECTRONIC CONFIGURATION:
The general electronic configuration of alkali metals may
be represented by [noble gas] ns1 where n = 2 to
7
PHYSICAL PROPERTIES:
(1) SOFTNESS OF ALKALI METALS:
Except Francium (Fr) all
the alkali metals are soft, malleable and metallic lusture when they are
freshly cut due to oscillation of loosely binded electrons. Down the group softness increases due to the
decreasing of cohesive energy hence Li is the
hardest element while Cs is the softest element
in first group.
All the alkali elements are silvery white solid. The
silvery luster of alkali metals is due to the presence of highly mobile
electrons of the metallic lattice. There being only a single electron per atom,
These are highly malleable and ductile. the metallic
bonding is not so strong. As the result, the metals are soft in nature.
However, the softness increases with increase in atomic number due to
continuous decrease in metallic bond strength on account of an increase in
atomic size.
COHESIVE ENERGY: Cohesive energy is just reverse of atomization energy, magnitude is
same but sign is different. The (energy) force by which atoms or ions are bind
together in solid state called cohesive energy.
(2) ATOMIC AND
IONIC RADII:
The atoms of alkali metals have the largest size in their
respective periods. The atomic radii increase on moving down the group among
the alkali metals.
Li < Na
< K < Rb < Cs < Fr
REASON: On moving down the group a new shell is
progressively added. Although, the nuclear charge also increases down the group
but the effect of addition of new shells is more predominant due to increasing
screening effect of inner filled shell on the valence s-electrons. Hence the
atomic size increases in a group.
IONIC RADIUS: Alkali metals change into positively
charged ions by losing their valence electron. The size of cation is smaller
than parent atom of alkali metals. However, within the group the ionic radii
increase with increases in atomic number.
Li+ <
Na+ < K+ < Rb+ < Cs+ <
Fr+
HYDRATED RADIUS: The alkali metal ions get extensively
hydrated in aqueous solutions. Smaller the ion more is the extent or degree of
hydration. Thus, the ionic radii in aqueous solution follow the order
Li+ >
Na+ > K+ > Rb+ > Cs+ >
Fr+
The charge density on Li+ is higher in
comparison to other alkali metals due to which it is extensively hydrated.
(3) IONIZATION ENERGY (ENTHALPY):
The first
ionization energy of the alkali metals are the lowest as compared to the
elements in the other group. The ionization energy of alkali metals decreases
down the group.
REASON: The size of alkali metals is largest in
their respective period. So the outermost electron experiences less force of
attraction from the nucleus and hence can be easily removed.
The value of ionization energy decreases down the group
because the size of metal increases due to the addition of new shell along with
increase in the magnitude of screening effect.
(4) OXIDATION STATE:
The alkali metals show +1 oxidation state. The alkali metals
can easily loose their valence electron and change into uni-positive ions
REASON: Due to low ionization energy, the alkali
metals can easily lose their valence electron and gain stable noble gas
configuration. But the alkali metals cannot form M+2
ions as the magnitude of second ionization energy is very high.
ions as the magnitude of second ionization energy is very high.
(5) REDUCING PROPERTIES:
The alkali metals have low values of reduction potential
and therefore have a strong tendency to lose electrons and act as good reducing
agents. The reducing character increases from sodium to caesium. However lithium is the strongest reducing agent.
Li > Na > K > Rb > Cs > Fr
REASON: The alkali metals have low value of
ionization energy which decreases down the group and so can easily lose their
valence electron and thus act as good reducing agents. The reducing character of any metal is best measured
in terms of its electrode potential which among other things depends upon its
(1) Heat of vaporization
(2) Ionization
energy and
(3) Heat of
hydration.
Since Li+ ion has the smaller size, its heat
of hydration has the highest value. Therefore, among the alkali metals Li has
the highest negative electrode potential (E0 cell=3.05 volts) and
hence is the strongest reducing agent.
(6) ELECTROPOSITIVE CHARACTER:
On account of their low ionization energies, these metals
have a strong tendency to lose their valence electrons and thus change into
positive ions. Consequently, alkali metals are strongly electropositive or metallic in character. As this tendency for losing electrons
increases down the group, the electropositive character increases.
Li < Na
< K < Rb < Cs
(7) COLOUR: The
compounds of alkali metals are typically white
(8) MAGNETIC BEHAVIOR: The
compounds of alkali metals are diamagnetic. Superoxides of alkali metals are,
however, paramagnetic.
(9) HYDRATION:
Most of alkali metal salts dissolve in water. In solution alkali metal ions are
hydrated. Since Li+ ion is smallest in size it is most heavily hydrated. Salts
of lithium such as LiF, Li2CO3, and Li3PO4
are insoluble in water.
(10) MELTING AND BOILING POINTS:
The melting and boiling points of alkali metals are very
low because the intermetallic bonds in them are quite weak. And this decreases
with increase in atomic number with increases in atomic size.
(11) DENSITY:
The densities of alkali metals are quite low as compared
to other metals. Li, Na and K are even lighter than water. The density
increases from Li to Cs.
REASON: Due to their large size, the atoms of
alkali metals are less closely packed. Consequently have low density. On going
down the group, both the atomic size and atomic mass increase but the increase
in atomic mass compensates the bigger atomic size. As a result, the density of
alkali metals increases from Li to Cs. Potassium is however lighter than
sodium. It is probably due to an unusal increase in atomic size of potassium.
(12) NATURE OF BOND FORMATION:
All the alkali metals form ionic (electrovalent)
compounds. The ionic character increases from Li to Cs because the alkali
metals have low value of ionization energies which decreases down the group and
hence tendency to give electron increases to form electropositive ion.
(13) CONDUCTIVITY:
The alkali metals are good conductors of heat and
electricity. This is due to the presence of loosely held valence electrons
which are free to move throughout the metal structure.
(14) IONIC MOBILITY:
Ionic mobility of ion is inversely proportional to size
of hydrated ion
Size of the hydrated ion is = Li+(aq) > Na+(aq)
> K+(aq) > Rb+(aq) > Cs+(aq)
Order of ionic mobility = Li+(aq) < Na+(aq)
< K+(aq) < Rb+(aq) < Cs+(aq)
(15) PHOTOELECTRIC EFFECT:
Alkali metals (except Li) exhibit photoelectric effect (A
phenomenon of emission of electrons from the surface of metal when light falls
on them). The ability to exhibit photoelectric effect is due to low value of
ionization energy of alkali metals. Li does not emit photoelectrons due to high
value of ionization energy. Generally K,
Rb, Cs used photoelectric cell (mainly Cs).
(16) FLAME COLOURATION:
The alkali metals and their salts impart a characteristic
colour to flame
REASON: On heating an alkali metal or its salt
(especially chlorides due to its more volatile nature in a flame), the
electrons are excited easily to higher energy levels because of absorption of
energy. When these electrons return to their ground states, they emit extra
energy in form of radiations which fall in the visible region thereby imparting
a characteristic colour to the flame.
SUMMARY TABLE:
:ILLUSTRATIVE EXAMPLES:
ILLUSTRATIVE EXAMPLES (1): Why are Group 1 elements
called alkali metals?
SOLUTION: The Group 1 elements are called alkali
metals because they form water soluble hydroxides.
ILLUSTRATIVE EXAMPLE (2): What is the most reactive
alkali metal and why?
SOLUTION: The most reactive alkali metal is cesium
due to its lowest first ionization enthalpy and lowest electronegativity.
ILLUSTRATIVE EXAMPLE (3): The alkali metals have low
densities. Explain. ?
SOLUTION: The alkali metals have low densities due
to their large atomic sizes. In fact, Li, Na and K are even lighter than water.
ILLUSTRATIVE EXAMPLE (4): Write three general
characteristics of the elements of s-block of the periodic table which
distinguish them from the elements of the other blocks.
SOLUTION: The three general characteristics are:
(1) The compounds of the s-block elements are
mainly ionic.
(2) The valency is equal to the group number.
(3) Due to low ionization energy, s-block
elements are good reducing agents.
ILLUSTRATIVE EXAMPLE (5) which alkali metal is most
abundant in earth’s crust?
SOLUTION: Sodium is the most abundant alkali
metal in the earth’s crust.
ILLUSTRATIVE EXAMPLE (6): Why is the density of
potassium less than sodium?
SOLUTION: This is due to abnormal increase in the
atomic size of potassium.
ILLUSTRATIVE EXAMPLE (7): Why is lithium the
strongest reducing agent in the periodic table?
SOLUTION: The Eo value (reduction
potential) depends on the three factors i.e. sublimation, ionization and
hydration enthalpies. With the small size of its ion lithium has the lightest
hydration enthalpy which accounts for its high negative Eo value and
its reducing power
ILLUSTRATIVE EXAMPLE (8): Name the metal which floats
on water without any apparent reaction with it.
SOLUTION: Lithium
ILLUSTRATIVE EXAMPLE (9): Which is softer – Na or K
and why?
SOLUTION: Potassium is softer than sodium due to
weak metallic bonding because of the large size of K atoms.
ILLUSTRATIVE EXAMPLE (10): What makes sodium highly
reactive?
SOLUTION: Low ionization enthalpy, strongly
electropositive nature, tendency to attain noble gas configuration by the loss
of one valence electron makes sodium highly reactive.
ILLUSTRATIVE EXAMPLE (11): Alkali metals impart colour to Bunsen flame due to
(A) The
presence of one electron in their outermost orbital
(B) Low
ionization energies
(C) Their
softness
(D) Their
reducing nature
SOLUTION: (B)
ILLUSTRATIVE EXAMPLE (12): The metallic lustre
exhibited by sodium is explained by
(A) Diffusion
of sodium ions
(B)Oscillation
of loose electrons
(C) Excitation
of free protons
(D) Existence
of body–centered cubic lattice
SOLUTION: (B)
ALKALI METALS - CHEMICAL PROPERTIES:
Continuous…..