Topics covered
(1)SOLUBILITY AND SOLUBILITY PRODUCT
(2) FACTER'S AFFECTING SOLUBILITY
(1) Effect of temperature on Solubility
(2) Effect of common ions on Solubility
(3) Effect of simultaneous on Solubility
(4) Effect of solvent on Solubility
(5) Effect of PH on Solubility
(i) Effect of PH on Solubility of metal hydroxide
(ii) Effect of PH on Solubility of salt of weak acid
(iii) Effect of PH on Solubility of salt of strong acid
(6) Effect of buffer solution on Solubility of salt
(7) Effect of complex formation on Solubility
SOLUBILITY(S):
Solubility of any substance (electrolyte) in solvent
represent its amount present in the given amount of solution in order to make
the solution saturated at a constant temperature.
MOLAR
SOLUBILITY:
Number of mole of a solute dissolved in one litre saturated
solution at constant temperature is called molar solubility.
Unit of solubility(s)=mole/litre
If solubility is equal or more than 0.1 then salt is
soluble.
If solubility is equal or less than 0.1 then salt is partially
soluble or sparingly soluble.
The solubility of substance in water may be classified
as.
(1)Molecular
Solubility: define for molecular solid, example glucose ,urea and other covalent soluble solid.
(2)Ionic Solubility: define for electrolyte
SOLUBILITY PRODUCT (Ksp):
It is the product of the ionic concentration of the ions
of binary solid electrolyte in saturated state at constant temperature.
(1) Let solubility of a compound Ax
By be s moles L–1 it means that if more than s moles are
dissolved in solvent (one litre ) only s
moles will be soluble, rest will be insoluble, following equilibrium is
established.
(2) According to law of mass action -
KSP
is called solubility product.
(3) At a certain temperature solubility product of a
compound is constant, it means that ions are formed in the manner that product
of their concentration is always a constant. However, it becomes clear that if
one of ions (A+ or B–) is added from outside, it would
tend to increase KSP because [A+] or [B–] has
increased, so that extra ions will react with other ions to convert into
insoluble part and it precipitates.
(4)
Case-1 If
It means it is a saturated solution.
Case-2 If
It means more solute can dissolved.
Case-3

It means solution is supersaturated i.e. precipitation will start to occurs.
RELATIONSHIP
BETWEEN SOLUBILITY AND SOLUBILITY PRODUCT:
The equilibrium for a saturated solution of a salt AxBy
may be expressed as.
Let the solubility of the salt AxBy in water at a particular temperature be ‘s’ moles per litre then
NOTE- (i) Ksp is
the equilibrium constant which depends upon temperature only.
(ii)In
general while calculating molar solubility we neglect hydrolysis of ions.
EXAMPLE(1): The solubility of CaCO3 is
0.03 g/L calculate Ksp ?
SOLUTION:
solubility in term of mole /liter is 0.03/100 mole/liter (M wt of CaCO3
is 100 u)
we know that
Ksp =(s)2
= (0.03/100)2
=9 x 10-8
(1)AB type of salts:
(2)AB2 or A2B type of salts:
(3)AB3 or A3B type of salt:
(4)A2B3 type of salts:
EXAMPLE(2): The solubility
product expression for La2(CO3)3 is Ksp =?
SOLUTION:First, write
the equilibrium expression. The subscript following each atom in the solid is the
number of ions of that atom in solution. The total charges must add to zero:
Given the information above and the equilibrium expression:
EXAMPLE(2): What will
be the solubility in moles/litre if solubility product of F3B2
is 2 × 10–30.
SOLUTION:
(2) FACTER'S AFFECTING SOLUBILITY
(1) Effect of temperature on Solubility: In General,most case Solubility increases on increasing temperature.However we must follow two case
(1) in endothermic reactions


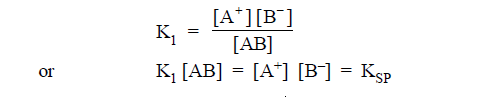











No comments:
Post a Comment