Law of Definite Proportions: Compounds are
consistent chemical combinations of atoms that can be expressed as:
(i) Ratio of
masses
(ii) Ratio of atoms
(iii) Ratio of moles of atoms
(iv) Percent
composition
ILLUSTRATIVE EXAMPLE (1): Calculate the percent Nitrogen in Dinitrogen (N2O)
Monoxide?
SOLUTION:
ILLUSTRATIVE EXAMPLE (2): What is the % composition of each
element in (Mg(OH)2) magnesium hydroxide?
SOLUTION:
ILLUSTRATIVE EXAMPLE (3): Haemoglobin contains 0.33% of Iron by weight. The
molecular weight of it is approx. 67200. The numbers of iron atoms (Atomic wt
of Fe=56 u) present in one molecule of Haemoglobin are?
SOLUTION:
ILLUSTRATIVE EXAMPLE (4): The hydrated salt, Na2SO4.nH2O
undergoes 55.9% loss in weight on heating and becomes anhydrous. The value of n
will be?
SOLUTION:
ILLUSTRATIVE EXAMPLE (5): Air contain 20 % O2
by volume. How many cm³ of air will be required for oxidation of 100 cm³(ml) of
acetylene?
SOLUTION:
Since air contain 20 % oxygen by volume then amount of
air required to react with 100cc/ml of C2H2
Try yourself:
Exercise (4): Two oxides of metal contain 72.4 and 70 of metal
respectively if formula of 2nd oxide is M2O3 find that of
the first.


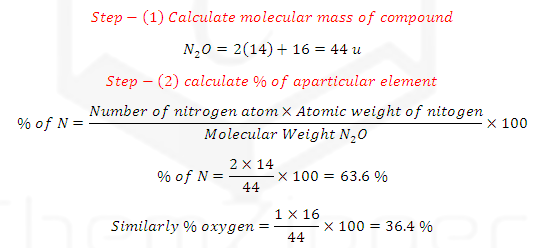

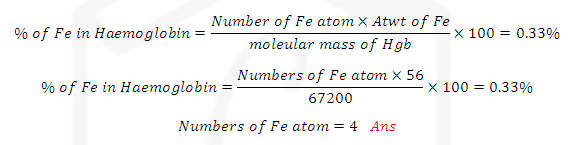



No comments:
Post a Comment