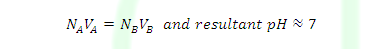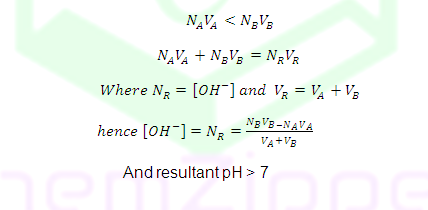ILLUSTRATIVE EXAMPLE:
Give the answers of
following questions when 20 ml of acetic acid (CH3COOH)
is titrated with 0.10 M NaOH the (Given that Ka=2×10-5).
(A) Write out the reactions
and equilibrium expression associated with Ka.
(B) Calculate the PH when:
(1) 20 ml of 0.10M CH3COOH + 0.0 ml of 0.10M NaOH
(2) 20 ml of 0.10M CH3COOH + 5.0 ml of 0.10M NaOH
(3) 20 ml of 0.10M CH3COOH + 10 ml of 0.10M NaOH
(4) 20 ml of 0.10M CH3COOH + 15 ml of 0.10M NaOH
(5) 20 ml of 0.10M CH3COOH + 19 ml of 0.10M NaOH
(6) 20 ml of 0.10M CH3COOH + 20 ml of 0.10M NaOH
(7) 20 ml of 0.10M CH3COOH + 21 ml of 0.10M NaOH
(8) 20 ml of 0.10M CH3COOH + 25 ml of 0.10M NaOH
(9) 20 ml of 0.10M CH3COOH + 20 ml of 0.10M NaOH
SOLUTION:
(A) Write out the reactions and equilibrium expression associated with Ka.
(B) Calculate the PH when:
|
S.N.
|
Given
condition
|
comments
|
PH
|
|
1
|
20 ml of 0.10M CH3COOH
+ 0.0
ml of 0.10 ml NaOH
|
WA, PH=1/2(Pka-logC)
|
2.85
|
|
2
|
20 ml of 0.10M CH3COOH
+ 5.0
ml of 0.10 ml NaOH
|
ABS,
PH=PKa+ log[S]\[A]
|
4.22
|
|
3
|
20 ml of 0.10M CH3COOH + 10 ml of 0.10 ml NaOH
|
BB , PH=PKa
Half of equivalent point
|
4.70
|
|
4
|
20 ml of 0.10M CH3COOH
+ 15
ml of 0.10 ml NaOH
|
ABS,
PH=PKa+ log[S]\[A]
|
5.17
|
|
5
|
20 ml of 0.10M CH3COOH
+ 19
ml of 0.10 ml NaOH
|
ABS,
PH=PKa+ log[S]\[A]
|
5.98
|
|
6
|
20 ml of 0.10M CH3COOH + 20 ml of 0.10 ml NaOH
|
SH, PH=7+
1/2(Pka-logC)
Equivalent point
|
8.7
|
|
7
|
20 ml of 0.10M CH3COOH
+ 21
ml of 0.10 ml NaOH
|
Strong
Base
|
11.39
|
|
8
|
20 ml of 0.10M CH3COOH
+ 25
ml of 0.10 ml NaOH
|
Strong
Base
|
12.04
|
|
9
|
20 ml of 0.10M CH3COOH
+ 30
ml of 0.10 ml NaOH
|
Strong
Base
|
12.30
|
(C) Sketch the titration
curve for this titration.