The titration of HCl (aq) with a
standardized NaOH solution illustrated the titration of strong acid by a strong
base.
The molecular and net ionic equation
is.
Case (1): At the start point before any titrant has
been added the receiving flask contains only 0.10 M HCl and 50 ml. Because it
is strong acid so
Case (2): After starting but before equivalent point.
Case (3): At equivalent point
Case (4): Before equivalent point
TITRATION SUMMARY TABLE:
|
S.N.
|
Volume of
HCl Taken
|
Volume
of NaOH
|
PH
|
|
|
1
|
50.0 ml (In ml)
And 0.10 M
|
0.0 (In ml)
And 0.10M
|
1.0
|
|
|
2
|
|
10
|
1.17
|
|
|
3
|
|
20
|
1.36
|
|
|
4
|
|
30
|
1.60
|
NAVA>NBVB
|
|
5
|
|
40
|
1.95
|
|
|
6
|
(Vertical Over)
|
45
|
2.27
|
|
|
7
|
|
49
|
2.99
|
|
|
8
|
50.0 ml (In ml)
And 0.10 M
|
50
|
7.0
|
NAVA=NBVB
|
|
9
|
|
51
|
11
|
NAVA<NBVB
|
|
10
|
|
60
|
11.95
|
|
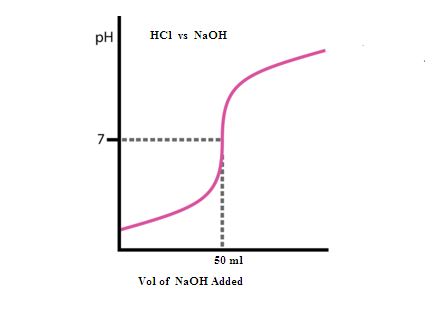
GRAPHICAL REPRESENTATION:
ILLUSTRATIVE EXAMPLE: Find the pH of following titrations:
(A) 500 ml, 0.10 M HCl + 500 ml 0.10 M Ca(OH)2
(B) 400 ml, M/200 Ca(OH)2 + 400 ml M/50 HNO3
ANSWERS KEY:
(A): PH=12.6989 (B): PH=2.6
(A): PH=12.6989 (B): PH=2.6


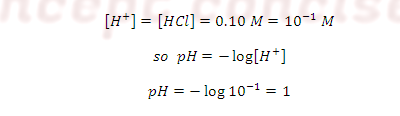

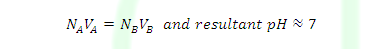
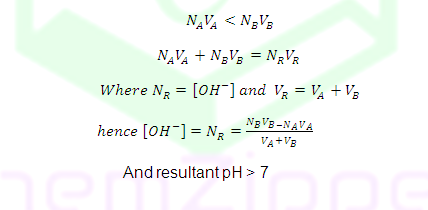
No comments:
Post a Comment