Guanidine is strongest
organic nitrogenous compound with the formula HNC(NH2)2.
Guanidine is analogue of carbonic acid. That is, the C=O group in carbonic acid is
replaced by a C=NH
group,
and each OH
is replaced by a NH2 group.
A guanidine group also
appears in larger organic molecules, including on the side chain of arginine
(a basic amino acid).
It is a colourless solid
that dissolves in polar solvents. It is a strong base that is used in the
production of plastics and explosives. It is found in urine as a normal product
of protein metabolism.
Basicity of Guanidine:
Guanidine is the strongest base among neutral
compounds:
The remarkable basicity of guanidine is
attributed to the fact that the positive charge on
the guanidinium ion is delocalized equally
over the three nitrogen atoms, as shown by
these three equivalent resonating structures:
Basicity of nitrogen can
be increased by attachment to pi-donors (NH2) group. These two
pi-donating NH2
groups donate electron density to the (pi-accepting) C=NH.
IIT UPDATE:
QUESTION:
SOLUTION: (B)
IIT UPDATE:
QUESTION:
SOLUTION: (B)



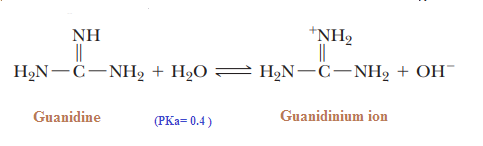


No comments:
Post a Comment