(1) Primary
condition of cannizaro reaction is absence of alpha hydrogen in aldehyde but
(CH3)2CH-CHO gives cannizaro reaction although it has one alpha
hydrogen.
(2) CCl3-CHO
does not give cannizaro reaction while it has no alpha hydrogen it give halo form
reaction.
(3) The overall
order of the reaction is usually 3.
(4) The Cannizzaro reaction takes place very slowly when electron-donating groups are present. But the reaction occurs at faster rates when electron withdrawing groups are present.
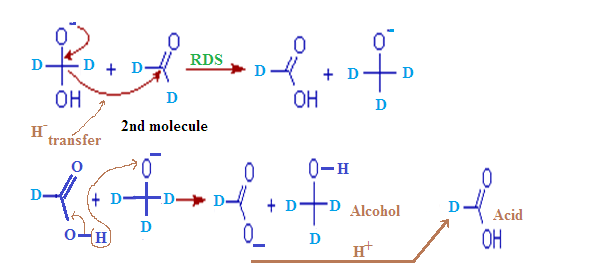
(5) Transfer of hydride is rate determining step.
(6) In cannizaro reaction kinetic isotopic effect is observed
Step -(1):
Step-(2):



No comments:
Post a Comment