(1) As Asymmetrical ketones,
that group migrate which is better able to supply electron (ERG). Thus order of
ease of migration is ….
OR
The
substituent’s which can stabilize the positive charge can migrate readily. The
migratory aptitude of various substituents is approximately:
3o-Alkyl > Cyclohexyl > 2o- Alkyl
> Benzyl > Aryl > 1o - Alkyl > Methyl
(2) The electron
withdrawing groups (-I groups) on peroxy acids enhance the rate of the
reaction.
(3) As the rearrangement is a concerted process,
the configuration of the migrating chiral substituent is retained.
(4) In case of
aldehydes, usually the hydrogen atom is migrated preferentially and thus by
furnishing carboxylic acids. But formates are also produced when the migrating
group is other than the hydrogen. This is possible when the other substituent
is a tertiary alkyl group or electron rich vinyl or aryl group.
-H > 3o-Alkyl > 2o-
Alkyl > Benzyl > Aryl > 1o - Alkyl > Methyl
(5) One of the
competing reactions is the formation of epoxide when a double bond is present
in the molecule especially at low temperatures in neutral solvents.
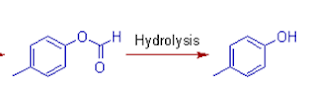
(6) The aldehyde is
oxidized to formate due to preferential migration of aryl group. But it
undergoes hydrolysis under the reaction conditions to yield a phenol.
(7) As illustrated
below, the aldehyde group is oxidized to carboxylic acid due to preferential
migration of the hydride ion. The aryl group with electronegative halogen
groups has less migratory aptitude. Remember the groups which can stabilize
positive charge possess greater migratory aptitude.
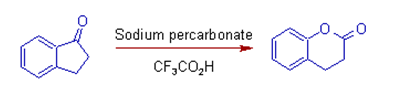
(8) The greater
migratory aptitude of aryl group over the -CH2 group can be observed
in the following example.
(9) The -CH2
group is migrated preferentially in the following reaction. The -CH-CF3
group has less migratory aptitude due to electron withdrawing nature.
(10) The lactone
formed can be reduced to a dihydric alcohol.





No comments:
Post a Comment