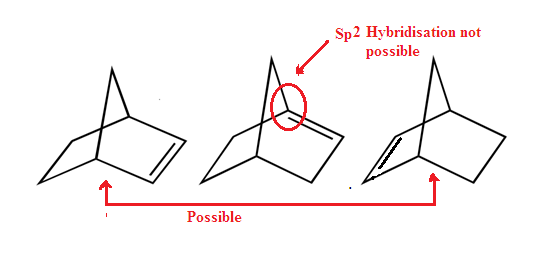
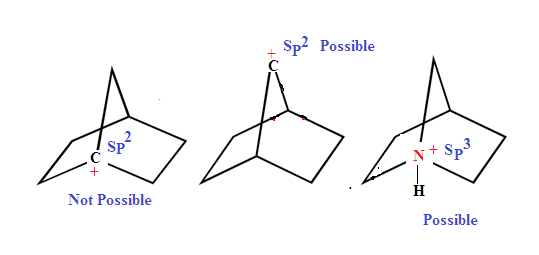
According to
Bredt’s rule a bridgehead carbon atom of bicyclo compound cannot be sp2
hybridised or in other word a bridgehead carbon atom cannot be form double bond.
unless the ring that contains at least eight atoms
In Other word Bredt's rule state that planarity is not possible at bridge head carbon of bicyclo compound till one of the ring size is (8) eight member or more.
ILLUSTRATIVE EXAMPLE: Bredt's use for the predicting major and minor product of elimination reactions.




No comments:
Post a Comment