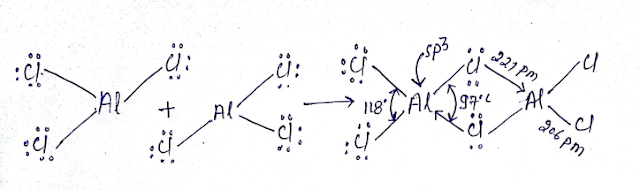
3C-4e BOND or 3C-4e BRIDGE BOND:
Al2Cl6 Dimmerised by 3C-4e bond bridge bond:
Al2Cl6 is neither hypovalent nor hypovalent rather its octet is complete. We will used MOT here it cannot act as Lewis acid due to crowding in spite having vacant d orbital’s however Alcl3 act as Lewis acid.
Al2Cl6 contains six bond having two bridge bond(3c-4e) and four bond is (2C-2e)
Boron do not formed bridge bond because boron experience steric crowding.


No comments:
Post a Comment