CF3-, CCl3-, CBr3- CI3-
We are expecting the acidic strength haloform acids asCHF3, CHCl3, CHBr3, CHI3 in decreasing order. Because Fluorine is most electronegative atom so it would be stabilize CF3- more, as electronegativity decreases from F to I the stability of conjugate -ve ion would be but that is not correct the actual order isCHCl3 > CHF3 > CHBr3 > CHI3.
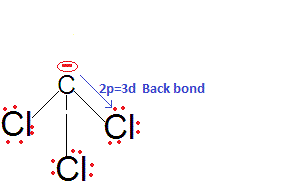
This is because there is effective back bonding in CCl3-and hence the negative charge partially gets stabilised by back donation to the vacant 3d orbitals of Cl. Thus, CHCl3 is a stronger acid than CHF3 and also among them due to 2pπ-3dπ back bonding.
The acidic strengths of the other three haloforms can be compared the inductive effects of their anions. F is very electronegative and hence stabilises the negative charge on the C atom. So, CHF3 is a better acid than CHBr3, and the least acidic is CHI3.
The overall acidic strength order is:
CHCl3 > CHF3 > CHBr3 > CHI3.

No comments:
Post a Comment