The part f energy which is converted into usefull work called Gibb's free energy or Gibb's function.
Energy (H) = Useful work (G) + Non useful (TS)
We van not calculate absolute value of 'G' so we calculate change in Gibb's free energy.
∆G = ∆H -∆TS
∆G = ∆H - (∆TS + T∆S). ......(1)
Standard Gibb's energy (∆G°) change at standard condition is 1 bar and 298 K
∆G° = ∆H° -∆TS° .......(2)
Relation between ∆G° and Equilibrium constant (K):

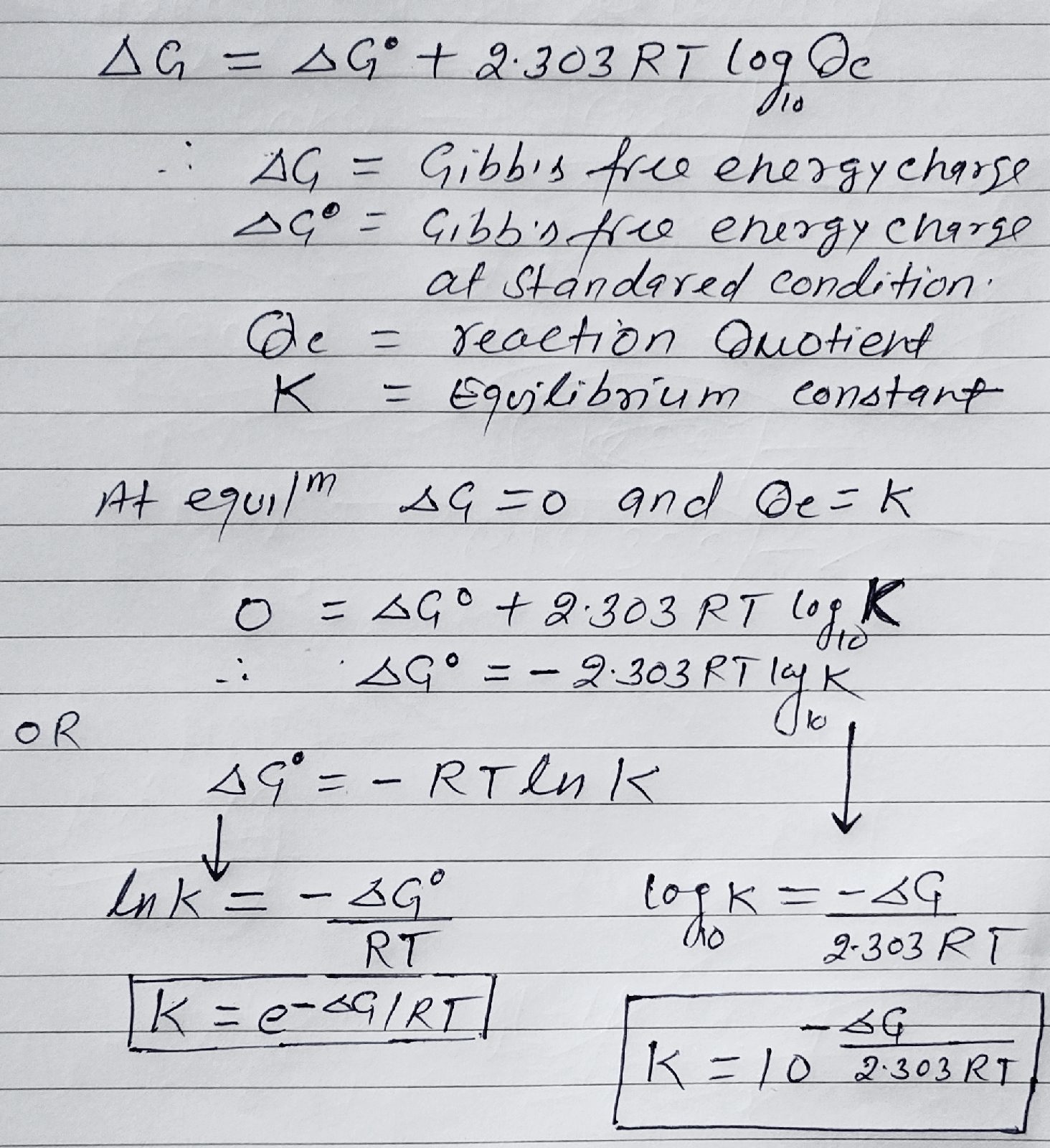
No comments:
Post a Comment