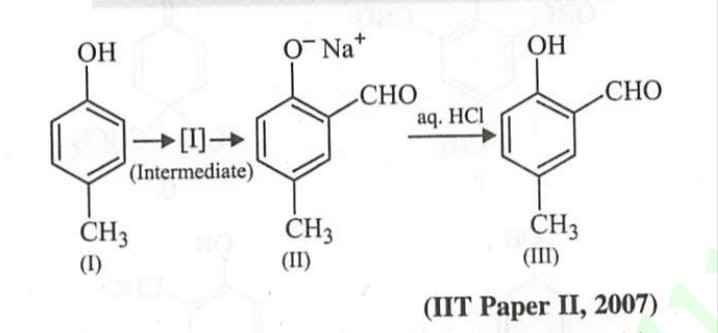
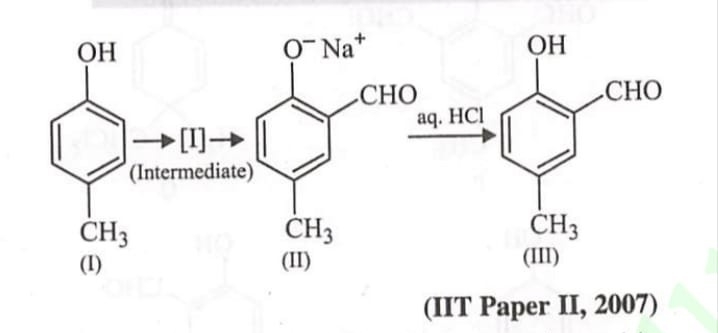
⇒It is the laboratory method for the preparation of ethers.
⇒It is a nucleophilic substitution reaction and proceeds through the SN2 mechanism.
⇒ Order of reactivity of primary halide is CH3X > CH3CH2X > CH3CH2CH2X.
⇒ Tendency of alkyl halide to undergo elimination is 3º > 2º > 1°.
⇒ For better yield alkyl halide should be primary and alkoxide should be secondary or tertiary.