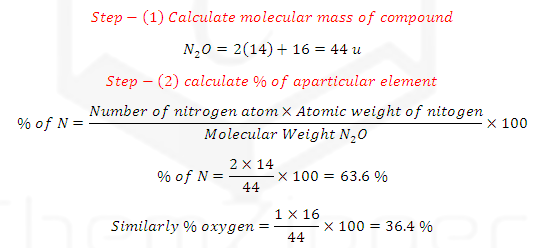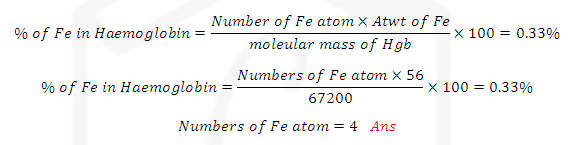(1)
Matter is made up of extremely small, indivisible particles called atoms.
(2)
Atom of same substance are identical in all respect i.e. they posses same size,
shape, mass, chemical properties etc.
(3)
Atom of different substances are different in all respect i.e. they posses
different shape, size, mass and chemical properties etc.
(4)
Atom is the smallest particle that takes part in chemical reactions.
(5)
Atom of different elements may combine with each other in a fixed, simple,
whole number ratio to form compound atoms.
(6)
Atom can neither be created nor destroyed i.e. atoms are indestructible.
Limitations of Dalton’s theory:
The
main failures of Dalton’s atomic theory are:
(1)
Atom was no more indivisible. It is made up of various sub-atomic
particles like electrons, proton and neutron etc.
(2)
It failed to explain how atoms of different elements differ from each other.
(3)
It failed to explain how and why atoms of elements combine with each other to
form compound atoms or molecules.
(4)
It failed to explain the nature of forces that bind together different atoms in
molecules.
(5)
It failed to explain Gay Lussac’s law of combining volumes.
(6)
It did not make any distinction between ultimate particle of an element that
takes part in reaction (atoms) and the ultimate particle that has independent
existence (molecules).
Modern Atomic theory:
(1) Atom is no longer supposed to be
indivisible. Atom has a complex structure and is composed of sub-atomic
particles such as electrons protons and neutrons.
(2)
Atom of the same element may not be similar in all respects e.g. isotopes.
(3)
Atom of different elements may be similar in one or more respects e.g. isobars.
(4)
Atom is the smallest unit which takes part in chemical reactions.
(5)
The ratio in which atoms unite may be fixed and integral but may not be simple.
e.g. In sugar molecules C12H22O11 the ratio of C, H and O atoms is 12:22:11 which is not
simple.
(6)
Atom of one element can be changed into atoms of other element for e.g.
transmutation.
(7)
Mass of atom can be changed in energy.
(E=MC2) According to Einstein mass energy relationship, mass and
energy are inter-convertible. Thus atom is no longer indestructible.
ILLUSTRATIVE
EXAMPLE: An important postulate of Dalton’s atomic theory is:
(A) an atom contains electrons, protons and
neutrons
(B) atom can neither be created nor destroyed nor
divisible
(C) all the atoms of an element are not identical
(D) all the elements are available in nature in the
form of atoms
SOLUTION: The statement written in (B) is about law
of mass conservation which is true for all chemical reaction. Hence (B) is
correct.