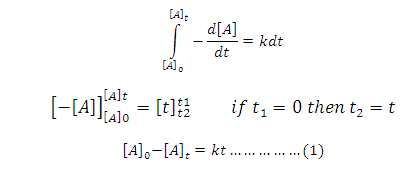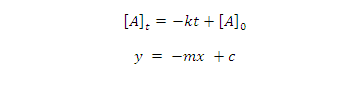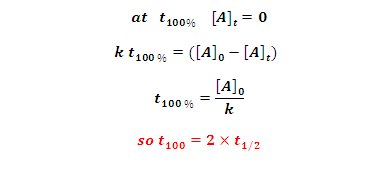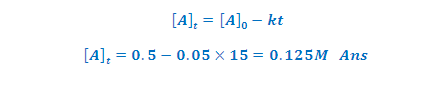Consider a
general chemical reaction its follow zero order kinetics
[A] 0 =initial concentration of reactant A
[A]t = concentration of after time t
[B]t = concentration of product B after
time t
On
Integration of above equation:
On rearrangement
equation (1) give equation of straight line
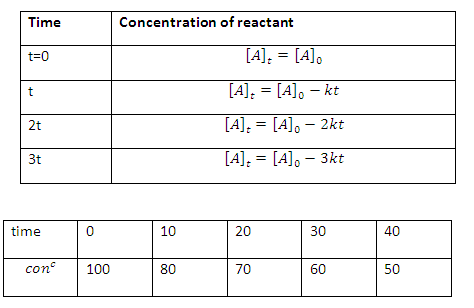
Note: Concentration
of reactant after regular interval of time will constitute an arithmetical
progression (AP)
Similarly
concentration of product B after time t
Unit of rate constant: K= Mol/litre second
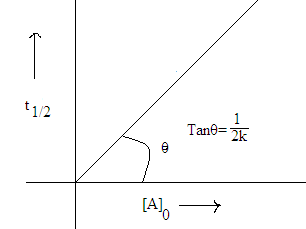
Half life period: Time required converting half of the reactant to product i.e.
life of zero order reaction may give as
Imp note: In zero order reaction average rate
of reaction is equal to instantaneous rate of reaction
100% completion of time: It is time in which reactant
completely converted into product.
ILLUSTRATIVE EXAMPLE (1): The graph show decomposition of Ammonia on Pt surface it initial concentration of ammonia
is 0.1 M then calculate the time required for the 40% completion of reaction.
SOLUTION:
ILLUSTRATIVE EXAMPLE (2): A certain zero order reaction has
k=0.025 M /S for disappearance of A what will be the concentration of A after 15 second if initial concentration is
0.5 M?
SOLUTION: concentration of A after time of t is [A]t