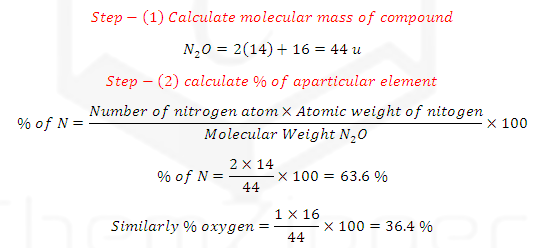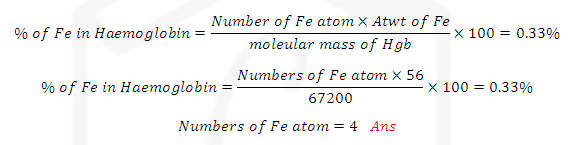(1) PERCENTAGE COMPOTION OF COMPOUNDS:
(2) EMPIRICAL FORMULA:
It
is the formula which expresses the smallest whole number ratio of the
constituent atom within the molecule. Empirical formula of different compound
may be same. So it may or may not represent the actual formula of the molecule.
It can be deduced by knowing the weight % of the entire constituent element
with their atomic masses for the given compound.
For example: C6H12O6,
CH3COOH, HCHO All have same empirical formula CH2O, but
they are different.
The empirical formula of a compound can be determined by the
following steps:
Step-(1)
Write the name of detected elements in column-1 present in the compound.
Step-(2)
Write the corresponding atomic mass in column-2.
Step-(2)
Write the experimentally determined percentage composition by weight of each
element present in the compound in column-3.
Step-(2)
Divide the percentage of each element by its atomic weight to get the relative
number of atoms of each element in column-4.
Step-(2)
Divide each number obtained for the respective elements in step (3) by the
smallest number among those numbers so as to get the simplest ratio in
column-5.
Step-(2)
If any number obtained in step (4) is not a whole number then multiply all the
numbers by a suitable integer to get whole number ratio. This ratio will be the
simplest ratio of the atoms of different elements present in the compound.
Empirical formula of the compound can be written with the help of this ratio in
column-6.
ILLUSTRATIVE EXAMPLE (1): A compound
contains C =71.23%, H = 12.95% and O = 15.81%. What is the empirical formula
of the compound?
SOLUTION:
ILLUSTRATIVE EXAMPLE (2): The
simplest formula of a compound containing 50% of element X (Atomic mass = 10)
and 50% of the element Y (Atomic mass = 20) is:
(A)
XY (B) X2Y
(C) XY2 (D) X2Y3
SOLUTION:
ILLUSTRATIVE EXAMPLE (3): A compound of carbon, hydrogen and nitrogen
contains these elements in the ratio 9:1:3.5. Calculate the empirical formula.
If its molecular mass is 108, what is the molecular formula?
SOLUTION
Empirical formula = C3H4N
Empirical formula mass = (3 ´ 12) + (4 ´ 1) + 14 = 54
Thus, molecular formula of the compound
= 2 ´ empirical formula = 2 ´ (C3H4N
)= C6H8N2
ILLUSTRATIVE EXAMPLE (4): 2.38 gm of uranium was heated strongly in a
current of air. The resulting oxide weighed 2.806 g. Determine the empirical
formula of the oxide. (At. mass U = 238; O = 16).
SOLUTION:
Step 1: To
calculate the percentage of uranium and oxygen in the oxide.
Step 2: To calculate the empirical formula
ILLUSTRATIVE EXAMPLE (5): Chemical analysis of a carbon compound gave
the following percentage composition by weight of the elements present. Carbon
10.06%, hydrogen 0.84%, chlorine 89.10%. Calculate the empirical formula of the
compound.
SOLUTION:
Step 1: Percentage of the elements present
Step 2: Dividing the
percentage compositions by the respective atomic weights of the elements
Step 3: Dividing
each value in step 2 by the smallest number among them to get simple atomic
ratio
Step 4: Ratio
of the atoms present in the molecule C : H :
Cl
1 :
1 : 3
The empirical formula of the compound is C1H1Cl3
o r CHCl3.
ILLUSTRATIVE EXAMPLE (6): A carbon compound
on analysis gave the following percentage composition. Carbon 14.5%, hydrogen 1.8%,
chlorine 64.46%, oxygen 19.24%. Calculate the empirical formula of the compound
SOLUTION:
Step 1: Percentage
composition of the elements present in the compound.
Step 2: Dividing
by the respective atomic weights
Step 3: Dividing
the values in step 2 among them by the smallest number.
Step 4: Multiplication
by a suitable integer to get whole number ratio.
Thus the
simplest ratio of the atoms of different elements in the compound.
C : H : Cl : O = 2 : 3 : 3 : 2
(1) MOLECULAR FORMULA:
The
formula which represents the actual number of each individual atom in any molecule
is known as molecular formula. For certain compounds the molecular formula and
the empirical formula may be same.
Molecular
formula= (Empirical formula) n
Molecular
Weight = Empirical Weight * n
If
the vapour density of the substance is known, its molecular weight can be
calculated by using the equation.
Molecular
Weight= 2* Vapour Density
ILLUSTRATIVE EXAMPLE (6): The empirical formula of a compound is . Its
molecular weight is 90. Calculate the molecular formula of the compound.
(Atomic weights C = 12, H = 1, O = 16)
SOLUTION:
Empirical
formula =CH2O
Empirical
formula weight = (12 + 2 + 16) = 30
The molecular formula = (CH2O)3=C3H6O3
ILLUSTRATIVE EXAMPLE (7): Certain non metal X forms two oxides I and II. The mass % of oxygen in
1st X4O5 is 43.7, which is same as that of X in 2nd oxide.
Find the formula of 2nd oxide.
SOLUTION:
Try yourself:
Exercise (1): A crystalline hydrated salt, on being rendered
anhydrous, loses 45.6% of its mass. The percentage composition of the anhydrous
salt is: Al = 10.5%, K = 15.1%, S =24.8% and oxygen = 49.6%. Find the empirical
formula of the anhydrous and crystalline hydrated salt. [K = 39; Al = 27; S = 32; O = 16; H =
1]
Exercise (2): A colourless crystalline compound
has the following percentage composition: Sulphur 24.24%, nitrogen 21.21%,
hydrogen 6.06% and the rest is oxygen. Determine the empirical formula of the
compound. If the molecular mass is 132, what is the molecular formula of the
compound? Name the compound if it is found to be sulphite.
Exercise (3) A gaseous hydrocarbon contains 85.7% carbon and 14.3%
hydrogen.
1 litre of the hydrocarbon weighs 1.26 g at NTP. Determine the molecular
formula of the hydrocarbon.
Answers Keys:
Exercise (1) Empirical formula of anhydrous salt = KAS2O8
Hydrated salt composition; % anhydrous part = 54.4% and % H2O = 45.6%;
Empirical formula of hydrated salt = KAIS2O8.12H2O.
Exercise
(2) Empirical formula SN2H8O4;
Molecular formula = SN2H8O4; Name – Ammonium
sulphite (NH4)2SO3.