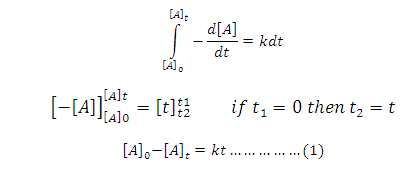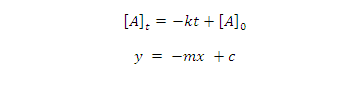A chemical
reaction is said to be of first order reaction if its rate is determined by the
change of one of the concentration term only.
Variation o f concentration with time:
Consider a
general chemical reaction its follow first order kinetics
Let [A] 0 =initial concentration of reactant A
[A]t = concentration of after time t
[B]t = concentration of product B after
time t
On
Integration of above equation
On rearrangement equation (1) give equation of straight line
We can also write equation (1) as
Case (1):
# On increasing angle (slop) the value of reaction constant increases hence k3>k2> k1
Case (2):
Case (3):
Case (4):
[A]t/[A]0 Vs t :
Characteristic of 1st
order:
(1) Concentration of reactant left after
regular time interval of time will constitute a geometrical progression (GP).
(2) A first order reaction takes infinite
time for completion.
(3) All radioactive disintegrations are
examples of first order reaction
(4) Decomposing of H2O2 , Ester and Inversion of sugar are examples of first order reactions
(4) Decomposing of H2O2 , Ester and Inversion of sugar are examples of first order reactions
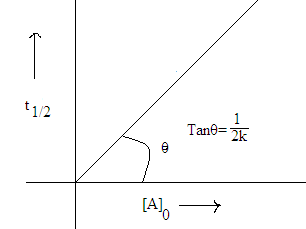
(5) Half life time of first order
reaction does not depend upon initial concentration.
Similarly concentration of product B after time t
Graph show increase in concentration of [B] and [A] both as exponential manner.
Half life time of first Order reaction:
Natural life time of first order reaction: